The dynamics of QuantiFERON-TB gold in-tube conversion and reversion in a cohort of South African adolescents
- PMID: 25562578
- PMCID: PMC4384770
- DOI: 10.1164/rccm.201409-1704OC
The dynamics of QuantiFERON-TB gold in-tube conversion and reversion in a cohort of South African adolescents
Abstract
Rationale: Interferon-γ release assays are used to diagnose tuberculosis infection. In developed countries, high rates of reversion following conversion have been described.
Objectives: To assess QuantiFERON TB Gold In-Tube test (QFT) conversion and reversion dynamics in a tuberculosis-endemic setting.
Methods: Adolescents aged 12-18 years residing near Cape Town were recruited. Tuberculin skin tests (TSTs) and QFTs were performed at baseline and after 2 years of follow up. Half of the participants had TST and QFT performed at additional time points. Participants were observed for incident tuberculosis disease for up to 5 years.
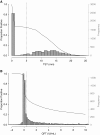
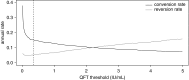
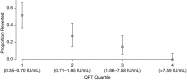
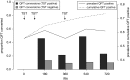
Measurements and main results: Among 5,357 participants, 2,751 (51.4%) and 2,987 (55.8%) had positive QFT and TST results, respectively, at baseline. Annualized QFT and TST conversion risks were 14.0 and 13.0%, respectively, and reversion risks were 5.1 and 4.1%, respectively. Concordance was excellent for conversions (κ = 0.74), but poor for reversions (κ = 0.12). Among recent QFT converters, the magnitude of the QFT value was strongly inversely associated with risk of reversion (P < 0.0001). When longitudinal QFT data were analyzed in a cross-sectional manner, the annual risk of infection was 7.3%, whereas inclusion of reversions in the analysis showed that the actual risk of infection was 14.0%. Incident tuberculosis was 8-fold higher among QFT reverters than in participants with all negative QFT results (1.47 vs. 0.18 cases/100 person-years, P = 0.011).
Conclusions: In this tuberculosis-endemic setting, annual risk of infection was extremely high, whereas QFT and TST conversion concordance was higher and QFT reversion rates were lower than reported in low-burden settings.
Keywords: adolescents; epidemiology; interferon-γ release assays; tuberculin skin tests; tuberculosis.
Figures
References
-
- Machingaidze S, Verver S, Mulenga H, Abrahams DA, Hatherill M, Hanekom W, Hussey GD, Mahomed H. Predictive value of recent QuantiFERON conversion for tuberculosis disease in adolescents. Am J Respir Crit Care Med. 2012;186:1051–1056. - PubMed
-
- Mahomed H, Hawkridge T, Verver S, Geiter L, Hatherill M, Abrahams DA, Ehrlich R, Hanekom WA, Hussey GD SATVI Adolescent Study Team. Predictive factors for latent tuberculosis infection among adolescents in a high-burden area in South Africa. Int J Tuberc Lung Dis. 2011;15:331–336. - PubMed
-
- ClinicalTrials.govA randomized, placebo controlled, partially blinded phase II study to evaluate safety, immunogenicity, and prevention of infection with Mycobacterium tuberculosis of AERAS-404 and BCG revaccination in healthy adolescents (040-404) [accessed 2014 Apr 30]. Available from: http://clinicaltrials.gov/ct2/show/NCT02075203
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

