Distinct brain systems mediate the effects of nociceptive input and self-regulation on pain
- PMID: 25562688
- PMCID: PMC4285399
- DOI: 10.1371/journal.pbio.1002036
Distinct brain systems mediate the effects of nociceptive input and self-regulation on pain
Abstract
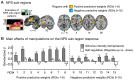
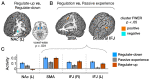
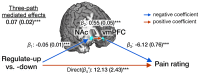
Cognitive self-regulation can strongly modulate pain and emotion. However, it is unclear whether self-regulation primarily influences primary nociceptive and affective processes or evaluative ones. In this study, participants engaged in self-regulation to increase or decrease pain while experiencing multiple levels of painful heat during functional magnetic resonance imaging (fMRI) imaging. Both heat intensity and self-regulation strongly influenced reported pain, but they did so via two distinct brain pathways. The effects of stimulus intensity were mediated by the neurologic pain signature (NPS), an a priori distributed brain network shown to predict physical pain with over 90% sensitivity and specificity across four studies. Self-regulation did not influence NPS responses; instead, its effects were mediated through functional connections between the nucleus accumbens and ventromedial prefrontal cortex. This pathway was unresponsive to noxious input, and has been broadly implicated in valuation, emotional appraisal, and functional outcomes in pain and other types of affective processes. These findings provide evidence that pain reports are associated with two dissociable functional systems: nociceptive/affective aspects mediated by the NPS, and evaluative/functional aspects mediated by a fronto-striatal system.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Comment in
-
Pain: a distributed brain information network?PLoS Biol. 2015 Jan 6;13(1):e1002037. doi: 10.1371/journal.pbio.1002037. eCollection 2015 Jan. PLoS Biol. 2015. PMID: 25562782 Free PMC article.
-
Pain: reappraising pain.Nat Rev Neurosci. 2015 Mar;16(3):124. doi: 10.1038/nrn3919. Epub 2015 Jan 29. Nat Rev Neurosci. 2015. PMID: 25630993 No abstract available.
-
Towards a taxonomy of pain modulations.Trends Cogn Sci. 2015 Apr;19(4):180-2. doi: 10.1016/j.tics.2015.02.007. Epub 2015 Mar 4. Trends Cogn Sci. 2015. PMID: 25745857
References
-
- Gross JJ, Munoz RF (1995) Emotion regulation and mental-health. Clin Psychol-Sci Pr 2: 151–164.
-
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD (2002) Rethinking feelings: an FMRI study of the cognitive regulation of emotion. J Cogn Neurosci 14: 1215–1229. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

