Influenza A (H1N1) virus infection triggers severe pulmonary inflammation in lupus-prone mice following viral clearance
- PMID: 25563403
- PMCID: PMC4324011
- DOI: 10.1016/j.jaut.2014.12.003
Influenza A (H1N1) virus infection triggers severe pulmonary inflammation in lupus-prone mice following viral clearance
Abstract
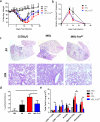
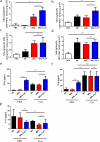
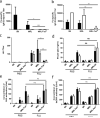
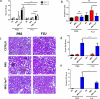
Each year, up to one fifth of the United States population is infected with influenza virus. Although mortality rates are low, hundreds of thousands are hospitalized each year in the United States. Specific high risk groups, such as those with suppressed or dysregulated immune systems, are at greater danger for influenza complications. Respiratory infections are a common cause of hospitalizations and early mortality in patients with systemic lupus erythematosus (SLE); however, whether this increased infection risk is a consequence of the underlying dysregulated immune background and/or immunosuppressing drugs is unknown. To evaluate the influenza immune response in the context of lupus, as well as assess the effect of infection on autoimmune disease in a controlled setting, we infected lupus-prone MRL/MpJ-Fas(lpr) mice with influenza virus A PR/8/34 H1N1. Interestingly, we found that Fas(lpr) mice generated more influenza A virus specific T cells with less neutrophil accumulation in the lung during acute infection. Moreover, Fas(lpr) mice produced fewer flu-specific IgG and IgM antibodies, but effectively cleared the virus. Further, increased extrinsic apoptosis during influenza infection led to a delay in autoimmune disease pathology with decreased severity of splenomegaly and kidney disease. Following primary influenza A infection, Fas(lpr) mice had severe complications during the contraction and resolution phase with widespread severe pulmonary inflammation. Our findings suggest that influenza infection may not exacerbate autoimmune pathology in mice during acute infection as a direct result of virus induced apoptosis. Additionally, autoimmunity drives an enhanced antigen-specific T cell response to clear the virus, but persisting pulmonary inflammation following viral clearance may cause complications in this lupus animal model.
Keywords: Influenza; Lupus; MRL-Fas(lpr); Pulmonary inflammation; SLE.
Published by Elsevier Ltd.
Figures

Similar articles
-
Negative role of colony-stimulating factor-1 in macrophage, T cell, and B cell mediated autoimmune disease in MRL-Fas(lpr) mice.J Immunol. 2004 Oct 1;173(7):4744-54. doi: 10.4049/jimmunol.173.7.4744. J Immunol. 2004. PMID: 15383612
-
Deficient leptin signaling ameliorates systemic lupus erythematosus lesions in MRL/Mp-Fas lpr mice.J Immunol. 2014 Feb 1;192(3):979-84. doi: 10.4049/jimmunol.1301685. Epub 2014 Jan 3. J Immunol. 2014. PMID: 24391210 Free PMC article.
-
Cyclosporin A alleviates influenza A (H1N1) virus-induced chronic pulmonary inflammation through decreasing IFN-γ-producing T lymphocytes.Virol J. 2025 Jul 25;22(1):255. doi: 10.1186/s12985-025-02887-4. Virol J. 2025. PMID: 40713716 Free PMC article.
-
Impact of Obesity on Influenza A Virus Pathogenesis, Immune Response, and Evolution.Front Immunol. 2019 May 10;10:1071. doi: 10.3389/fimmu.2019.01071. eCollection 2019. Front Immunol. 2019. PMID: 31134099 Free PMC article. Review.
-
[Endogenous retroviral sequences as a factor in the pathogenesis of systemic lupus erythematosus].Hautarzt. 1996 Jul;47(7):502-9. doi: 10.1007/s001050050460. Hautarzt. 1996. PMID: 8926164 Review. German.
Cited by
-
Autoimmunity in 2015.Clin Rev Allergy Immunol. 2016 Aug;51(1):110-9. doi: 10.1007/s12016-016-8576-1. Clin Rev Allergy Immunol. 2016. PMID: 27422713 Review.
-
Coronavirus infection (SARS-CoV-2) in obesity and diabetes comorbidities: is heat shock response determinant for the disease complications?Diabetol Metab Syndr. 2020 Jul 16;12:63. doi: 10.1186/s13098-020-00572-w. eCollection 2020. Diabetol Metab Syndr. 2020. PMID: 32690985 Free PMC article.
-
Viruses and Autoimmunity: A Review on the Potential Interaction and Molecular Mechanisms.Viruses. 2019 Aug 19;11(8):762. doi: 10.3390/v11080762. Viruses. 2019. PMID: 31430946 Free PMC article. Review.
-
COVID-19 in patients with systemic lupus erythematosus: lessons learned from the inflammatory disease.Transl Res. 2021 Jun;232:13-36. doi: 10.1016/j.trsl.2020.12.007. Epub 2020 Dec 19. Transl Res. 2021. PMID: 33352298 Free PMC article. Review.
References
-
- Dasaraju PV, Liu C. Infections of the Respiratory System. In: Baron S, editor. Medical Microbiology. Galveston (TX): 1996. - PubMed
-
- Zambon MC. The pathogenesis of influenza in humans. Reviews in medical virology. 2001;11:227–41. - PubMed
-
- Powell TJ, Brown DM, Hollenbaugh JA, Charbonneau T, Kemp RA, Swain SL, et al. CD8+ T cells responding to influenza infection reach and persist at higher numbers than CD4+ T cells independently of precursor frequency. Clinical immunology. 2004;113:89–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

