Receptor-interacting protein kinase 3 contributes to abdominal aortic aneurysms via smooth muscle cell necrosis and inflammation
- PMID: 25563840
- PMCID: PMC4329096
- DOI: 10.1161/CIRCRESAHA.116.304899
Receptor-interacting protein kinase 3 contributes to abdominal aortic aneurysms via smooth muscle cell necrosis and inflammation
Abstract
Rationale: Depletion of medial smooth muscle cell (SMC) is a major pathological characteristic of abdominal aortic aneurysm (AAA), although the mechanism by which these cells are eliminated remains incompletely understood. We reasoned that necroptosis, a recently described form of necrosis mediated by receptor-interacting protein kinase 3 (RIP3), may contribute to AAA pathology through the induction of SMC death and the significant production of inflammatory cytokines.
Objective: To test the hypothesis that RIP3-mediated necroptosis is actively involved in aneurysm pathogenesis.
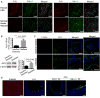
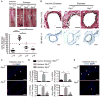
Methods and results: RIP3 and RIP1 levels were found to be elevated in human AAAs, most noticeably in SMCs. Elevations of RIP3 and SMC necrosis were also observed in the elastase-induced mouse model of AAAs. Deletion of one or both copies of Rip3 prevented AAA formation. By transplanting Rip3(+/-) aortae to Rip3(+/+) mice, we demonstrated that reduced Rip3 expression in arterial wall was the primary cause of aneurysm resistance. In vitro, adenoviral overexpression of RIP3 was sufficient to trigger SMC necroptosis. Protein kinase C-delta contributed to tumor necrosis factor-α-induced SMC necroptosis by regulating Rip3 expression. Furthermore, Rip3 deficiency impaired tumor necrosis factor-α-induced inflammatory gene expression in aortic SMCs, which was at least in part because of attenuation of p65 Ser536 phosphorylation. In vivo, the lack of RIP3 diminished activation of p65 in SMCs, implicating a necrosis independent function of RIP3 in aneurysms.
Conclusions: Enhanced RIP3 signaling in aneurysmal tissues contributes to AAA progression by causing SMC necroptosis, as well as stimulating vascular inflammation, and therefore may serve as a novel therapeutic target for AAA treatment.
Keywords: Rip3 protein, mouse; aortic aneurysm, abdominal; apoptosis; myocytes, smooth muscle; necrosis; nuclear factor kappa B; protein kinase C-delta.
© 2015 American Heart Association, Inc.
Figures






References
-
- Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase b) suppresses development of experimental abdominal aortic aneurysms. The Journal of Clinical Investigation. 2000;105:1641–1649. - PMC - PubMed
-
- Shimizu K, Mitchell RN, Libby P. Inflammation and cellular immune responses in abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2006;26:987–994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

