The apoptotic mechanism of action of the sphingosine kinase 1 selective inhibitor SKI-178 in human acute myeloid leukemia cell lines
- PMID: 25563902
- PMCID: PMC4352591
- DOI: 10.1124/jpet.114.219659
The apoptotic mechanism of action of the sphingosine kinase 1 selective inhibitor SKI-178 in human acute myeloid leukemia cell lines
Abstract
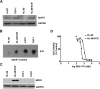
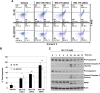
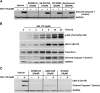
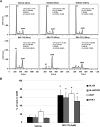
We previously developed SKI-178 (N'-[(1E)-1-(3,4-dimethoxyphenyl)ethylidene]-3-(4-methoxxyphenyl)-1H-pyrazole-5-carbohydrazide) as a novel sphingosine kinase-1 (SphK1) selective inhibitor and, herein, sought to determine the mechanism-of-action of SKI-178-induced cell death. Using human acute myeloid leukemia (AML) cell lines as a model, we present evidence that SKI-178 induces prolonged mitosis followed by apoptotic cell death through the intrinsic apoptotic cascade. Further examination of the mechanism of action of SKI-178 implicated c-Jun NH2-terminal kinase (JNK) and cyclin-dependent protein kinase 1 (CDK1) as critical factors required for SKI-178-induced apoptosis. In cell cycle synchronized human AML cell lines, we demonstrate that entry into mitosis is required for apoptotic induction by SKI-178 and that CDK1, not JNK, is required for SKI-178-induced apoptosis. We further demonstrate that the sustained activation of CDK1 during prolonged mitosis, mediated by SKI-178, leads to the simultaneous phosphorylation of the prosurvival Bcl-2 family members, Bcl-2 and Bcl-xl, as well as the phosphorylation and subsequent degradation of Mcl-1. Moreover, multidrug resistance mediated by multidrug-resistant protein1 and/or prosurvival Bcl-2 family member overexpression did not affect the sensitivity of AML cells to SKI-178. Taken together, these findings highlight the therapeutic potential of SKI-178 targeting SphK1 as a novel therapeutic agent for the treatment of AML, including multidrug-resistant/recurrent AML subtypes.
Copyright © 2015 by The American Society for Pharmacology and Experimental Therapeutics.
Figures

Similar articles
-
Development of SKI-349, a dual-targeted inhibitor of sphingosine kinase and microtubule polymerization.Bioorg Med Chem Lett. 2020 Oct 15;30(20):127453. doi: 10.1016/j.bmcl.2020.127453. Epub 2020 Jul 28. Bioorg Med Chem Lett. 2020. PMID: 32736077 Free PMC article.
-
Sphingosine kinase inhibitors decrease viability and induce cell death in natural killer-large granular lymphocyte leukemia.Cancer Biol Ther. 2015;16(12):1830-40. doi: 10.1080/15384047.2015.1078949. Cancer Biol Ther. 2015. PMID: 26252351 Free PMC article.
-
SphK1 inhibitor II (SKI-II) inhibits acute myelogenous leukemia cell growth in vitro and in vivo.Biochem Biophys Res Commun. 2015 May 15;460(4):903-8. doi: 10.1016/j.bbrc.2015.03.114. Epub 2015 Mar 28. Biochem Biophys Res Commun. 2015. PMID: 25824043
-
Concurrent targeting Akt and sphingosine kinase 1 by A-674563 in acute myeloid leukemia cells.Biochem Biophys Res Commun. 2016 Apr 15;472(4):662-8. doi: 10.1016/j.bbrc.2016.02.094. Epub 2016 Feb 23. Biochem Biophys Res Commun. 2016. PMID: 26920060
-
Targeting sphingosine kinase 1 induces MCL1-dependent cell death in acute myeloid leukemia.Blood. 2017 Feb 9;129(6):771-782. doi: 10.1182/blood-2016-06-720433. Epub 2016 Dec 12. Blood. 2017. PMID: 27956387 Free PMC article.
Cited by
-
Therapeutic potential of targeting sphingosine kinases and sphingosine 1-phosphate in hematological malignancies.Leukemia. 2016 Nov;30(11):2142-2151. doi: 10.1038/leu.2016.208. Epub 2016 Jul 27. Leukemia. 2016. PMID: 27461062 Review.
-
Combating Acute Myeloid Leukemia via Sphingosine Kinase 1 Inhibitor-Nanomedicine Combination Therapy with Cytarabine or Venetoclax.Pharmaceutics. 2024 Jan 31;16(2):209. doi: 10.3390/pharmaceutics16020209. Pharmaceutics. 2024. PMID: 38399263 Free PMC article.
-
Development of SKI-349, a dual-targeted inhibitor of sphingosine kinase and microtubule polymerization.Bioorg Med Chem Lett. 2020 Oct 15;30(20):127453. doi: 10.1016/j.bmcl.2020.127453. Epub 2020 Jul 28. Bioorg Med Chem Lett. 2020. PMID: 32736077 Free PMC article.
-
Pivotal role of mitophagy in response of acute myelogenous leukemia to a ceramide-tamoxifen-containing drug regimen.Exp Cell Res. 2019 Aug 15;381(2):256-264. doi: 10.1016/j.yexcr.2019.05.021. Epub 2019 May 18. Exp Cell Res. 2019. PMID: 31112736 Free PMC article.
-
Sphingosine kinase inhibitors decrease viability and induce cell death in natural killer-large granular lymphocyte leukemia.Cancer Biol Ther. 2015;16(12):1830-40. doi: 10.1080/15384047.2015.1078949. Cancer Biol Ther. 2015. PMID: 26252351 Free PMC article.
References
-
- Adan-Gokbulut A, Kartal-Yandim M, Iskender G, Baran Y. (2013) Novel agents targeting bioactive sphingolipids for the treatment of cancer. Curr Med Chem 20:108–122. - PubMed
-
- Baran Y, Gunduz U, Ural AU. (2006) Cross-resistance to cytosine arabinoside in human acute myeloid leukemia cells selected for resistance to vincristine. Exp Oncol 28:163–165. - PubMed
-
- Barboule N, Truchet I, Valette A. (2005) Localization of phosphorylated forms of Bcl-2 in mitosis: co-localization with Ki-67 and nucleolin in nuclear structures and on mitotic chromosomes. Cell Cycle 4:590–596. - PubMed
-
- Bayerl MG, Bruggeman RD, Conroy EJ, Hengst JA, King TS, Jimenez M, Claxton DF, Yun JK. (2008) Sphingosine kinase 1 protein and mRNA are overexpressed in non-Hodgkin lymphomas and are attractive targets for novel pharmacological interventions. Leuk Lymphoma 49:948–954. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

