Investigation of the Physico-Chemical Properties that Enable Co-Formulation of Basal Insulin Degludec with Fast-Acting Insulin Aspart
- PMID: 25563978
- PMCID: PMC4452210
- DOI: 10.1007/s11095-014-1614-x
Investigation of the Physico-Chemical Properties that Enable Co-Formulation of Basal Insulin Degludec with Fast-Acting Insulin Aspart
Abstract
Purpose: To study the self-association states of insulin degludec and insulin aspart alone and combined in pharmaceutical formulation and under conditions simulating the subcutaneous depot.
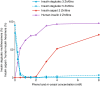
Methods: Formulations were made of 0.6 mM degludec at 3 and 5 Zn/6 insulin monomers, and 0.6 mM aspart (2 Zn/6 insulin monomers). Self-association was assessed using size-exclusion chromatography (SEC) monitored by UV and orthogonal reverse-phase chromatography.
Results: Simulating pharmaceutical formulation, degludec eluted as dihexamers, whereas aspart eluted as hexamers and monomers. Combining degludec at low zinc with aspart increased dihexamer content, indicating hybrid hexamer formation. At high zinc concentration, however, there was no evidence of this. Simulating the subcutaneous depot by removing preservative, degludec eluted as multihexamers and aspart as monomers. Aspart was incorporated into the multihexamer structures when combined with degludec at low zinc, but there was no such interaction with high-zinc degludec. SEC using progressively diluted concentrations of phenol and m-cresol showed that dissociation of aspart into monomers occurs before the formation of degludec multihexamers.
Conclusion: Insulins degludec and aspart can be combined without forming hybrid hexamers, but this combinability is dependent on zinc and preservative concentration, and requires that degludec is fully dihexameric before addition of aspart.
Figures



References
-
- Polonsky KS, Sturis J, Bell GI. Seminars in medicine of the beth Israel hospital, Boston. Non-insulin-dependent diabetes mellitus - a genetically programmed failure of the beta cell to compensate for insulin resistance. N Engl J Med. 1996;334(12):777–83. doi: 10.1056/NEJM199603213341207. - DOI - PubMed
-
- Krayenbühl C, Rosenberg T. Crystalline protamine insulin. Rep Steno Mem Hosp Nord Insulin lab. 1946;1:60–73.
-
- Lepore M, Pampanelli S, Fanelli C, Porcellati F, Bartocci L, Di Vincenzo A, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49(12):2142–8. doi: 10.2337/diabetes.49.12.2142. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

