Neurochemical and BOLD responses during neuronal activation measured in the human visual cortex at 7 Tesla
- PMID: 25564236
- PMCID: PMC4420878
- DOI: 10.1038/jcbfm.2014.233
Neurochemical and BOLD responses during neuronal activation measured in the human visual cortex at 7 Tesla
Abstract
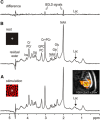
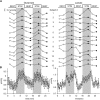
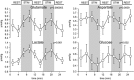
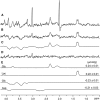
Several laboratories have consistently reported small concentration changes in lactate, glutamate, aspartate, and glucose in the human cortex during prolonged stimuli. However, whether such changes correlate with blood oxygenation level-dependent functional magnetic resonance imaging (BOLD-fMRI) signals have not been determined. The present study aimed at characterizing the relationship between metabolite concentrations and BOLD-fMRI signals during a block-designed paradigm of visual stimulation. Functional magnetic resonance spectroscopy (fMRS) and fMRI data were acquired from 12 volunteers. A short echo-time semi-LASER localization sequence optimized for 7 Tesla was used to achieve full signal-intensity MRS data. The group analysis confirmed that during stimulation lactate and glutamate increased by 0.26 ± 0.06 μmol/g (~30%) and 0.28 ± 0.03 μmol/g (~3%), respectively, while aspartate and glucose decreased by 0.20 ± 0.04 μmol/g (~5%) and 0.19 ± 0.03 μmol/g (~16%), respectively. The single-subject analysis revealed that BOLD-fMRI signals were positively correlated with glutamate and lactate concentration changes. The results show a linear relationship between metabolic and BOLD responses in the presence of strong excitatory sensory inputs, and support the notion that increased functional energy demands are sustained by oxidative metabolism. In addition, BOLD signals were inversely correlated with baseline γ-aminobutyric acid concentration. Finally, we discussed the critical importance of taking into account linewidth effects on metabolite quantification in fMRS paradigms.
Figures
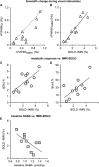
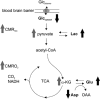
References
-
- Mangia S, Tkac I, Gruetter R, Van de Moortele PF, Maraviglia B, Ugurbil K. Sustained neuronal activation raises oxidative metabolism to a new steady-state level: evidence from 1H NMR spectroscopy in the human visual cortex. J Cereb Blood Flow Metab. 2007;27:1055–1063. - PubMed
-
- Mangia S, Tkac I, Logothetis NK, Gruetter R, Van de Moortele PF, Ugurbil K. Dynamics of lactate concentration and blood oxygen level-dependent effect in the human visual cortex during repeated identical stimuli. J Neurosci Res. 2007;85:3340–3346. - PubMed
-
- Schaller B, Mekle R, Xin L, Kunz N, Gruetter R. Net increase of lactate and glutamate concentration in activated human visual cortex detected with magnetic resonance spectroscopy at 7 Tesla. J Neurosci Res. 2013;91:1076–1083. - PubMed
-
- Schaller B, Xin L, O'Brien K, Magill AW, Gruetter R. Are glutamate and lactate increases ubiquitous to physiological activation? A (1)H functional MR spectroscopy study during motor activation in human brain at 7 Tesla. Neuroimage. 2014;93 Pt 1:138–145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

