A heterodimer of a VHH (variable domains of camelid heavy chain-only) antibody that inhibits anthrax toxin cell binding linked to a VHH antibody that blocks oligomer formation is highly protective in an anthrax spore challenge model
- PMID: 25564615
- PMCID: PMC4358291
- DOI: 10.1074/jbc.M114.627943
A heterodimer of a VHH (variable domains of camelid heavy chain-only) antibody that inhibits anthrax toxin cell binding linked to a VHH antibody that blocks oligomer formation is highly protective in an anthrax spore challenge model
Abstract
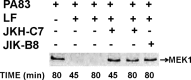
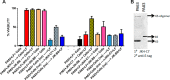
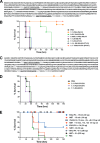
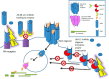
Anthrax disease is caused by a toxin consisting of protective antigen (PA), lethal factor, and edema factor. Antibodies against PA have been shown to be protective against the disease. Variable domains of camelid heavy chain-only antibodies (VHHs) with affinity for PA were obtained from immunized alpacas and screened for anthrax neutralizing activity in macrophage toxicity assays. Two classes of neutralizing VHHs were identified recognizing distinct, non-overlapping epitopes. One class recognizes domain 4 of PA at a well characterized neutralizing site through which PA binds to its cellular receptor. A second neutralizing VHH (JKH-C7) recognizes a novel epitope. This antibody inhibits conversion of the PA oligomer from "pre-pore" to its SDS and heat-resistant "pore" conformation while not preventing cleavage of full-length 83-kDa PA (PA83) by cell surface proteases to its oligomer-competent 63-kDa form (PA63). The antibody prevents endocytosis of the cell surface-generated PA63 subunit but not preformed PA63 oligomers formed in solution. JKH-C7 and the receptor-blocking VHH class (JIK-B8) were expressed as a heterodimeric VHH-based neutralizing agent (VNA2-PA). This VNA displayed improved neutralizing potency in cell assays and protected mice from anthrax toxin challenge with much better efficacy than the separate component VHHs. The VNA protected virtually all mice when separately administered at a 1:1 ratio to toxin and protected mice against Bacillus anthracis spore infection. Thus, our studies show the potential of VNAs as anthrax therapeutics. Due to their simple and stable nature, VNAs should be amenable to genetic delivery or administration via respiratory routes.
Keywords: Anthrax Toxin; Antibody; Antibody Engineering; Receptor; Toxin.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

