Establishing neural crest identity: a gene regulatory recipe
- PMID: 25564621
- PMCID: PMC4302844
- DOI: 10.1242/dev.105445
Establishing neural crest identity: a gene regulatory recipe
Abstract
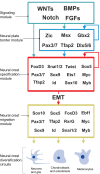
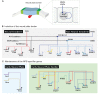
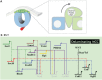
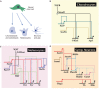
The neural crest is a stem/progenitor cell population that contributes to a wide variety of derivatives, including sensory and autonomic ganglia, cartilage and bone of the face and pigment cells of the skin. Unique to vertebrate embryos, it has served as an excellent model system for the study of cell behavior and identity owing to its multipotency, motility and ability to form a broad array of cell types. Neural crest development is thought to be controlled by a suite of transcriptional and epigenetic inputs arranged hierarchically in a gene regulatory network. Here, we examine neural crest development from a gene regulatory perspective and discuss how the underlying genetic circuitry results in the features that define this unique cell population.
Keywords: Gene regulation; Migration; Neural crest; Neural plate border; Signaling; Transcription factors.
© 2015. Published by The Company of Biologists Ltd.
Figures




References
-
- Agarwal P., Verzi M. P., Nguyen T., Hu J., Ehlers M. L., McCulley D. J., Xu S.-M., Dodou E., Anderson J. P., Wei M. L. et al. (2011). The MADS box transcription factor MEF2C regulates melanocyte development and is a direct transcriptional target and partner of SOX10. Development 138, 2555-2565 10.1242/dev.056804 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

