Tetraspanin CD9 and ectonucleotidase CD73 identify an osteochondroprogenitor population with elevated osteogenic properties
- PMID: 25564652
- PMCID: PMC4302994
- DOI: 10.1242/dev.113571
Tetraspanin CD9 and ectonucleotidase CD73 identify an osteochondroprogenitor population with elevated osteogenic properties
Abstract
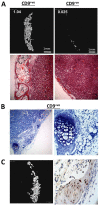
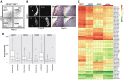
Cell-based bone regeneration strategies offer promise for traumatic bone injuries, congenital defects, non-union fractures and other skeletal pathologies. Postnatal bone remodeling and fracture healing provide evidence that an osteochondroprogenitor cell is present in adult life that can differentiate to remodel or repair the fractured bone. However, cell-based skeletal repair in the clinic is still in its infancy, mostly due to poor characterization of progenitor cells and lack of knowledge about their in vivo behavior. Here, we took a combined approach of high-throughput screening, flow-based cell sorting and in vivo transplantation to isolate markers that identify osteochondroprogenitor cells. We show that the presence of tetraspanin CD9 enriches for osteochondroprogenitors within CD105(+) mesenchymal cells and that these cells readily form bone upon transplantation. In addition, we have used Thy1.2 and the ectonucleotidase CD73 to identify subsets within the CD9(+) population that lead to endochondral or intramembranous-like bone formation. Utilization of this unique cell surface phenotype to enrich for osteochondroprogenitor cells will allow for further characterization of the molecular mechanisms that regulate their osteogenic properties.
Keywords: Osteoblast; Osteochondroprogenitor; Skeletal stem cell.
© 2015. Published by The Company of Biologists Ltd.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

