Stochastic modeling indicates that aging and somatic evolution in the hematopoetic system are driven by non-cell-autonomous processes
- PMID: 25564763
- PMCID: PMC4298364
- DOI: 10.18632/aging.100707
Stochastic modeling indicates that aging and somatic evolution in the hematopoetic system are driven by non-cell-autonomous processes
Abstract
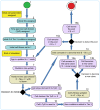
Age-dependent tissue decline and increased cancer incidence are widely accepted to be rate-limited by the accumulation of somatic mutations over time. Current models of carcinogenesis are dominated by the assumption that oncogenic mutations have defined advantageous fitness effects on recipient stem and progenitor cells, promoting and rate-limiting somatic evolution. However, this assumption is markedly discrepant with evolutionary theory, whereby fitness is a dynamic property of a phenotype imposed upon and widely modulated by environment. We computationally modeled dynamic microenvironment-dependent fitness alterations in hematopoietic stem cells (HSC) within the Sprengel-Liebig system known to govern evolution at the population level. Our model for the first time integrates real data on age-dependent dynamics of HSC division rates, pool size, and accumulation of genetic changes and demonstrates that somatic evolution is not rate-limited by the occurrence of mutations, but instead results from aged microenvironment-driven alterations in the selective/fitness value of previously accumulated genetic changes. Our results are also consistent with evolutionary models of aging and thus oppose both somatic mutation-centric paradigms of carcinogenesis and tissue functional decline. In total, we demonstrate that aging directly promotes HSC fitness decline and somatic evolution via non-cell-autonomous mechanisms.
Conflict of interest statement
The authors of this manuscript declare no conflict of interest.
Figures






References
-
- Serrano M, Blasco MA. Cancer and ageing: convergent and divergent mechanisms. Nat Rev Mol Cell Biol. 2007;8:715–722. - PubMed
-
- Hoeijmakers JHJ. DNA Damage, Aging, and Cancer. New England Journal of Medicine. 2009;361:1475–1485. - PubMed
-
- Vijg J, Busuttil RA, Bahar R, Dolle ME. Aging and genome maintenance. Ann N Y Acad Sci. 2005;1055:35–47. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

