Senescence from G2 arrest, revisited
- PMID: 25564883
- PMCID: PMC4353294
- DOI: 10.1080/15384101.2014.1000134
Senescence from G2 arrest, revisited
Abstract
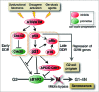
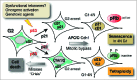
Senescence was classically defined as an irreversible cell cycle arrest in G1 phase (G1 exit) triggered by eroded telomeres in aged primary cells. The molecular basis of this G1 arrest is thought to be due to a DNA damage response, resulting in accumulation of the cyclin dependent kinase (Cdk) inhibitors p21 and p16 that block the inactivating phosphorylation of the retinoblastoma tumor suppressor pRb, thereby preventing DNA replication. More than a decade ago, several studies showed that p21 also mediates permanent DNA damage-induced cell cycle arrest in G2 (G2 exit) by inhibiting mitotic Cdk complexes and pRb phosphorylation. The idea that the senescence program can also be launched after G2 arrest has gained support from several recent publications, including evidence for its existence in vivo.
Keywords: Cdk inhibitors; DNA damage; G2/M checkpoint; p53; pRb; senescence; telomeres.
Figures

References
-
- Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev 2010; 24:2463-79; PMID:21078816; http://dx.doi.org/10.1101/gad.1971610 - DOI - PMC - PubMed
-
- Salama R, Sadaie M, Hoare M, Narita M. Cellular senescence and its effector programs. Genes Dev 2014; 28:99-114; PMID:24449267; http://dx.doi.org/10.1101/gad.235184.113 - DOI - PMC - PubMed
-
- Burkhart DL, Sage J. Cellular mechanisms of tumour suppression by the retinoblastoma gene. Nat Rev Cancer 2008; 8:671-82; PMID:18650841; http://dx.doi.org/10.1038/nrc2399 - DOI - PMC - PubMed
-
- Polager S, Ginsberg D. p53 and E2f: partners in life and death. Nat Rev Cancer 2009; 9:738-48; PMID:19776743; http://dx.doi.org/10.1038/nrc2718 - DOI - PubMed
-
- Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol 2008; 9:759-69; PMID:18813293; http://dx.doi.org/10.1038/nrm2514 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
