Regulation of B lymphocyte responses to Toll-like receptor ligand binding during diabetes prevention in non-obese diabetic (NOD) mice
- PMID: 25564999
- PMCID: PMC4598313
- DOI: 10.1111/1753-0407.12263
Regulation of B lymphocyte responses to Toll-like receptor ligand binding during diabetes prevention in non-obese diabetic (NOD) mice
Abstract
Background: Interactions between genetic risk factors and the environment drive type 1 diabetes (T1D). The system of Toll-like receptors (TLR) detects these environmental triggers; however, the target cell that intermediates these interactions to drive T1D remains unknown.
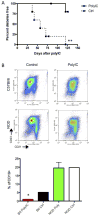
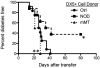
Methods: We investigated the effect of TLR pathway activation (myeloid differentiation primary response 88 [MyD88] vs TIR-domain-containing adapter-inducing interferon-β [TRIF]) on B cell subsets via flow cytometry, including their activation, survival, proliferation, and cytoskeletal mobilization. The effect of polyinosinic-polycytidylic acid (poly(I:C)) on diabetes development was addressed, including the B cell-dependent activation of diabetes-protective DX5+ cells, using genetic models and adoptive transfer.
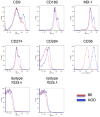
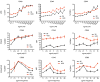
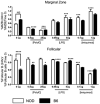
Results: B lymphocytes from non-obese diabetic (NOD) mice expressed enhanced levels of TLR-responsive proteins. Ex vivo analysis of B lymphocyte subsets demonstrated that TLR3 stimulation via TRIF deletes cells exhibiting a marginal zone phenotype, whereas MyD88-dependent ligands enhance their survival. In vivo, marginal zone B cells were activated by poly(I:C) and were unexpectedly retained in the spleen of NOD mice, in contrast with the mobilization of these cells in non-autoimmune mice, a phenotype we traced to defective actin cytoskeletal dynamics. These activated B cells mediated TLR3-induced diabetes protection.
Conclusions: Immunotherapies must account for both B cell location and activation, and these properties may differ in autoimmune and healthy settings.
Keywords: 1型糖尿病; B lymphocyte; B淋巴细胞; Toll-like receptor; Toll样受体; autoimmunity; innate immunity; type 1 diabetes; 先天性免疫; 自身免疫.
© 2015 Ruijin Hospital, Shanghai Jiaotong University School of Medicine and Wiley Publishing Asia Pty Ltd.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

