Organic carbamates in drug design and medicinal chemistry
- PMID: 25565044
- PMCID: PMC4393377
- DOI: 10.1021/jm501371s
Organic carbamates in drug design and medicinal chemistry
Abstract
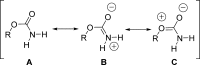
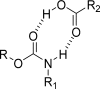
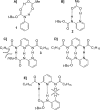
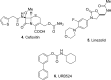
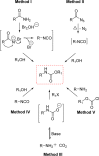
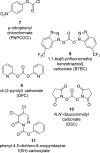
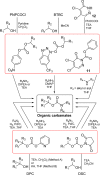
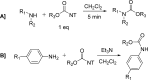
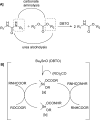
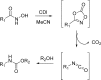
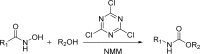
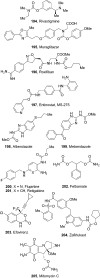
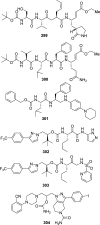
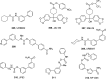
The carbamate group is a key structural motif in many approved drugs and prodrugs. There is an increasing use of carbamates in medicinal chemistry and many derivatives are specifically designed to make drug-target interactions through their carbamate moiety. In this Perspective, we present properties and stabilities of carbamates, reagents and chemical methodologies for the synthesis of carbamates, and recent applications of carbamates in drug design and medicinal chemistry.
Figures
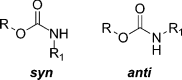
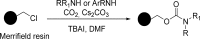
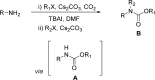
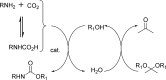
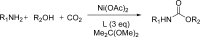
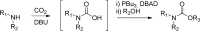

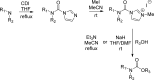
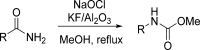
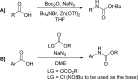
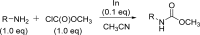
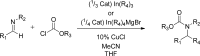


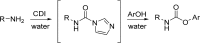
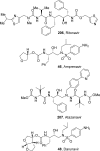
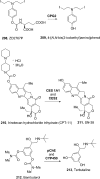
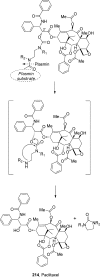
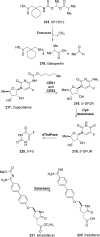
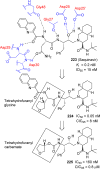
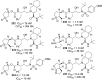
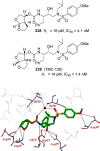
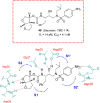
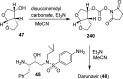
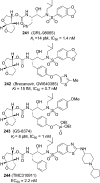
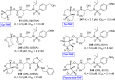
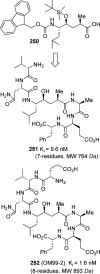
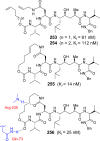
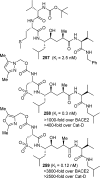
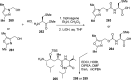
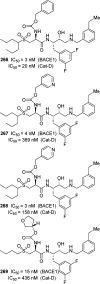
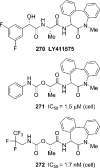
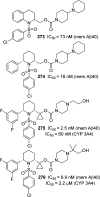
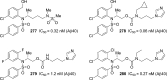
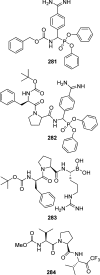
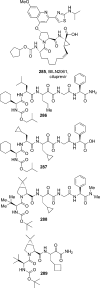
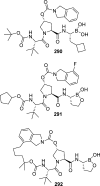
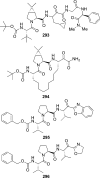
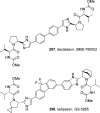
References
-
- Conradi R. A.; Hilgers A. R.; Ho N. F.; Burton P. S. The influence of peptide structure on transport across Caco-2 cells. II. Peptide bond modification which results in improved permeability. Pharm. Res. 1992, 9, 435–439. - PubMed
-
- Nielsen P. E.Pseudo-peptides in Drug Discovery; Wiley-VCH: Weinheim, Germany, 2004.
-
- Sewald N.; Jakubke H.-D.. Peptides: Chemistry and Biology; Wiley-VCH: Weinheim, Germany, 2002.
-
- Nowick J. S. What I have learned by using chemical model systems to study biomolecular structure and interactions. Org. Biomol. Chem. 2006, 4, 3869–3885. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

