Activation of human brown adipose tissue by a β3-adrenergic receptor agonist
- PMID: 25565203
- PMCID: PMC4298351
- DOI: 10.1016/j.cmet.2014.12.009
Activation of human brown adipose tissue by a β3-adrenergic receptor agonist
Abstract
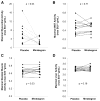
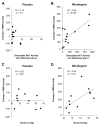
Increasing energy expenditure through activation of endogenous brown adipose tissue (BAT) is a potential approach to treat obesity and diabetes. The class of β3-adrenergic receptor (AR) agonists stimulates rodent BAT, but this activity has never been demonstrated in humans. Here we determined the ability of 200 mg oral mirabegron (Myrbetriq, Astellas Pharma, Inc.), a β3-AR agonist currently approved to treat overactive bladder, to stimulate BAT as compared to placebo. Mirabegron led to higher BAT metabolic activity as measured via (18)F-fluorodeoxyglucose ((18)F-FDG) using positron emission tomography (PET) combined with computed tomography (CT) in all twelve healthy male subjects (p = 0.001), and it increased resting metabolic rate (RMR) by 203 ± 40 kcal/day (+13%; p = 0.001). BAT metabolic activity was also a significant predictor of the changes in RMR (p = 0.006). Therefore, a β3-AR agonist can stimulate human BAT thermogenesis and may be a promising treatment for metabolic disease.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures

References
-
- Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–205. - PubMed
-
- Carey AL, Formosa MF, Van Every B, Bertovic D, Eikelis N, Lambert GW, Kalff V, Duffy SJ, Cherk MH, Kingwell BA. Ephedrine activates brown adipose tissue in lean but not obese humans. Diabetologia. 2013;56:147–155. - PubMed
-
- Cawthorne MA, Sennitt MV, Arch JR, Smith SA. BRL 35135, a potent and selective atypical beta-adrenoceptor agonist. Am J Clin Nutr. 1992;55(Suppl):252S–257S. - PubMed
-
- Chapple CR, Dvorak V, Radziszewski P, Van Kerrebroeck P, Wyndaele JJ, Bosman B, Boerrigter P, Drogendijk T, Ridder A, Van Der Putten-Slob I, Yamaguchi O Dragon Investigator Group. A phase II dose-ranging study of mirabegron in patients with overactive bladder. Int Urogynecol J. 2013;24:1447–1458. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

