Functional material features of Bombyx mori silk light versus heavy chain proteins
- PMID: 25565556
- PMCID: PMC4767016
- DOI: 10.1021/bm501667j
Functional material features of Bombyx mori silk light versus heavy chain proteins
Abstract
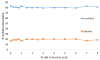
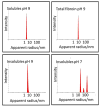
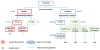
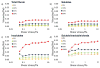
Bombyx mori (BM) silk fibroin is composed of two different subunits: heavy chain and light chain fibroin linked by a covalent disulfide bond. Current methods of separating the two silk fractions is complicated and produces inadequate quantities of the isolated components for the study of the individual light and heavy chain silks with respect to new materials. We report a simple method of separating silk fractions using formic acid. The formic acid treatment partially releases predominately the light chain fragment (soluble fraction) and then the soluble fraction and insoluble fractions can be converted into new materials. The regenerated original (total) silk fibroin and the separated fractions (soluble vs insoluble) had different molecular weights and showed distinctive pH stabilities against aggregation/precipitation based on particle charging. All silk fractions could be electrospun to give fiber mats with viscosity of the regenerated fractions being the controlling factor for successful electrospinning. The silk fractions could be mixed to give blends with different proportions of the two fractions to modify the diameter and uniformity of the electrospun fibers formed. The soluble fraction containing the light chain was able to modify the viscosity by thinning the insoluble fraction containing heavy chain fragments, perhaps analogous to its role in natural fiber formation where the light chain provides increased mobility and the heavy chain producing shear thickening effects. The simplicity of this new separation method should enable access to these different silk protein fractions and accelerate the identification of methods, modifications, and potential applications of these materials in biomedical and industrial applications.
Figures


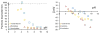


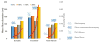

Similar articles
-
Fibroin silk proteins from the nonmulberry silkworm Philosamia ricini are biochemically and immunochemically distinct from those of the mulberry silkworm Bombyx mori.DNA Cell Biol. 2004 Mar;23(3):149-54. doi: 10.1089/104454904322964742. DNA Cell Biol. 2004. PMID: 15068584
-
Morphology and structure of electrospun mats from regenerated silk fibroin aqueous solutions with adjusting pH.Int J Biol Macromol. 2007 Oct 1;41(4):469-74. doi: 10.1016/j.ijbiomac.2007.06.006. Epub 2007 Jun 22. Int J Biol Macromol. 2007. PMID: 17689606
-
Electrospinning Bombyx mori silk with poly(ethylene oxide).Biomacromolecules. 2002 Nov-Dec;3(6):1233-9. doi: 10.1021/bm025581u. Biomacromolecules. 2002. PMID: 12425660
-
Applicability of biotechnologically produced insect silks.Z Naturforsch C J Biosci. 2017 Sep 26;72(9-10):365-385. doi: 10.1515/znc-2017-0050. Z Naturforsch C J Biosci. 2017. PMID: 28746045 Review.
-
Silk-based biomaterials.Biomaterials. 2003 Feb;24(3):401-16. doi: 10.1016/s0142-9612(02)00353-8. Biomaterials. 2003. PMID: 12423595 Review.
Cited by
-
Nanoscale probing of electron-regulated structural transitions in silk proteins by near-field IR imaging and nano-spectroscopy.Nat Commun. 2016 Oct 7;7:13079. doi: 10.1038/ncomms13079. Nat Commun. 2016. PMID: 27713412 Free PMC article.
-
Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine.Int J Mol Sci. 2018 Jan 30;19(2):407. doi: 10.3390/ijms19020407. Int J Mol Sci. 2018. PMID: 29385727 Free PMC article. Review.
-
Simple and Green Process for Silk Fibroin Production by Water Degumming.ACS Omega. 2025 Jan 3;10(1):272-280. doi: 10.1021/acsomega.4c05531. eCollection 2025 Jan 14. ACS Omega. 2025. PMID: 39829451 Free PMC article.
-
Micro and nano-scale compartments guide the structural transition of silk protein monomers into silk fibers.Nat Commun. 2022 Dec 21;13(1):7856. doi: 10.1038/s41467-022-35505-w. Nat Commun. 2022. PMID: 36543800 Free PMC article.
-
Systemic and Local Silk-Based Drug Delivery Systems for Cancer Therapy.Cancers (Basel). 2021 Oct 27;13(21):5389. doi: 10.3390/cancers13215389. Cancers (Basel). 2021. PMID: 34771557 Free PMC article. Review.
References
-
- Jin HJ, Kaplan DL. Nature. 2003;424:1057–1061. - PubMed
-
- Zuo B, Dai L, Wu Z. J Mater Sci. 2006;41:3357–3361.
-
- Mondal M, Trivedy K, Nirmal Kumar S. Caspian J Env Sci. 2007;5(2):63–76.
-
- Kim K, Jeong L, Park H, Shin S, Park W, Lee S, Kim T, Park Y, Seol Y, Lee Y, Ku Y, Rhyu I, Han S, Chung C. J Biotechnol. 2005;120:327–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

