How is national recipient hemovigilance conducted in the United States?
- PMID: 25565577
- PMCID: PMC4648700
- DOI: 10.1111/trf.12980
How is national recipient hemovigilance conducted in the United States?
Abstract
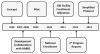
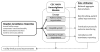
A national recipient hemovigilance system was introduced in the United States in 2010, when voluntary enrollment began as part of the National Healthcare Safety Network (NHSN) Hemovigilance Module. NHSN is a secure, Web-based surveillance system operated by the Centers for Disease Control and Prevention and used by US health care facilities to report a variety of patient safety information. The Hemovigilance Module is used for comprehensive monitoring of transfusion-related adverse events. Participating facilities can utilize analytic tools available within the module to identify opportunities for enhancing transfusion safety, evaluate the effectiveness of interventions, and compare facility specific transfusion-related data to aggregate national estimates. Data may be voluntarily shared by facilities with external partners for patient safety improvement initiatives and to fulfill reporting mandates. We describe the key characteristics of the Hemovigilance Module, highlight the benefits for participating facilities, and discuss the use of reported data for establishing national estimates of transfusion-associated adverse events to identify gaps in transfusion safety and opportunities for interventions. National hemovigilance systems are essential to recognize gaps in transfusion safety and identify opportunities for interventions to improve patient safety and outcomes.
Published 2015. This article is a U.S. Government work and is in the public domain in the USA.
Conflict of interest statement
Figures

Similar articles
-
Transfusion-related adverse reactions reported to the National Healthcare Safety Network Hemovigilance Module, United States, 2010 to 2012.Transfusion. 2015 Apr;55(4):709-18. doi: 10.1111/trf.12918. Epub 2014 Nov 5. Transfusion. 2015. PMID: 25371300 Free PMC article.
-
Transfusion-Transmitted Infections Reported to the National Healthcare Safety Network Hemovigilance Module.Transfus Med Rev. 2019 Apr;33(2):84-91. doi: 10.1016/j.tmrv.2019.01.001. Epub 2019 Jan 25. Transfus Med Rev. 2019. PMID: 30930009 Free PMC article.
-
Transfusion-related errors and associated adverse reactions and blood product wastage as reported to the National Healthcare Safety Network Hemovigilance Module, 2014-2022.Transfusion. 2024 Apr;64(4):627-637. doi: 10.1111/trf.17775. Epub 2024 Mar 12. Transfusion. 2024. PMID: 38476028 Free PMC article.
-
A Comparison of Transfusion-Related Adverse Reactions Among Apheresis Platelets, Whole Blood-Derived Platelets, and Platelets Subjected to Pathogen Reduction Technology as Reported to the National Healthcare Safety Network Hemovigilance Module.Transfus Med Rev. 2021 Apr;35(2):78-84. doi: 10.1016/j.tmrv.2021.03.003. Epub 2021 Apr 2. Transfus Med Rev. 2021. PMID: 33934903 Free PMC article. Review.
-
Hemovigilance in the United States of America.Vox Sang. 1998;74 Suppl 2:447-55. doi: 10.1111/j.1423-0410.1998.tb05455.x. Vox Sang. 1998. PMID: 9704480 Review.
Cited by
-
Transfusion reactions in pediatric and adolescent young adult haematology oncology and immune effector cell patients.EClinicalMedicine. 2020 Sep 9;26:100514. doi: 10.1016/j.eclinm.2020.100514. eCollection 2020 Sep. EClinicalMedicine. 2020. PMID: 32964199 Free PMC article.
-
Determining the true incidence of acute transfusion reactions: Active surveillance at a specialized liver center.Hematol Transfus Cell Ther. 2020 Oct-Dec;42(4):326-332. doi: 10.1016/j.htct.2019.09.006. Epub 2019 Nov 30. Hematol Transfus Cell Ther. 2020. PMID: 31838025 Free PMC article.
-
Evaluation of the National Healthcare Safety Network Hemovigilance Module for transfusion-related adverse reactions in the United States.Transfusion. 2019 Feb;59(2):524-533. doi: 10.1111/trf.15008. Epub 2018 Nov 14. Transfusion. 2019. PMID: 30427540 Free PMC article.
-
A multicentre study investigating vital sign changes occurring in complicated and uncomplicated transfusions.Vox Sang. 2018 Feb;113(2):160-169. doi: 10.1111/vox.12621. Epub 2017 Dec 25. Vox Sang. 2018. PMID: 29277907 Free PMC article.
-
Incidence of transfusion reactions: a multicenter study utilizing systematic active surveillance and expert adjudication.Transfusion. 2016 Oct;56(10):2587-2596. doi: 10.1111/trf.13730. Epub 2016 Jul 26. Transfusion. 2016. PMID: 27460200 Free PMC article.
References
-
- Robillard P, Nawej KI, Jochem K. The Quebec hemovigilance system: description and results from the first two years. Transfus Apher Sci. 2004;31:111–22. - PubMed
-
- Strong D, AuBuchon J, Whitaker B, Kuehnert M. Biovigilance initiatives. ISBT Science Series. 2008;3:77–84.
-
- Andreu G, Morel P, Forestier F, Debeir J, Rebibo D, Janvier G, Herve P. Hemovigilance network in France: organization and analysis of immediate transfusion incident reports from 1994 to 1998. Transfusion. 2002;42:1356–64. - PubMed
-
- Stainsby D, Jones H, Asher D, Atterbury C, Boncinelli A, Brant L, Chapman CE, Davison K, Gerrard R, Gray A, Knowles S, Love EM, Milkins C, McClelland DB, Norfolk DR, Soldan K, Taylor C, Revill J, Williamson LM, Cohen H, Group SS. Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfus Med Rev. 2006;20:273–82. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

