Transmyocardial revascularization devices: technology update
- PMID: 25565905
- PMCID: PMC4274152
- DOI: 10.2147/MDER.S51591
Transmyocardial revascularization devices: technology update
Abstract
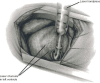
Transmyocardial laser revascularization (TMR) emerged as treatment modality for patients with diffuse coronary artery disease not amendable to percutaneous or surgical revascularization. The procedure entails the creation of laser channels within ischemic myocardium in an effort to better perfuse these areas. Currently, two laser devices are approved by the US Food and Drug Administration for TMR - holmium:yttrium-aluminum-garnet and CO2. The two devices differ in regard to energy outputs, wavelengths, ability to synchronize with the heart cycle, and laser-tissue interactions. These differences have led to studies showing different efficacies between the two laser devices. Over 50,000 procedures have been performed worldwide using TMR. Improvements in angina stages, quality of life, and perfusion of the myocardium have been demonstrated with TMR. Although several mechanisms for these improvements have been suggested, evidence points to new blood vessel formation, or angiogenesis, within the treated myocardium, as the major contributory factor. TMR has been used as sole therapy and in combination with coronary artery bypass grafting. Clinical studies have demonstrated that TMR is both safe and effective in angina relief long term. The objective of this review is to present the two approved laser devices and evidence for the safety and efficacy of TMR, along with future directions with this technology.
Keywords: angiogenesis; coronary artery disease; laser; revascularization.
Figures




References
-
- Mannheimer C, Camici P, Chester MR, et al. The problem of chronic refractory angina; report from the ESC Joint Study Group on the Treatment of Refractory Angina. Eur Heart J. 2002;23(5):355–370. - PubMed
-
- Jolicoeur EM, Granger CB, Henry TD, et al. Working Group Members Clinical and research issues regarding chronic advanced coronary artery disease: part I: Contemporary and emerging therapies. Am Heart J. 2008;155(3):418–434. - PubMed
-
- Jolicoeur EM, Ohman EM, Temple R, et al. Working Group Members Clinical and research issues regarding chronic advanced coronary artery disease part II: Trial design, outcomes, and regulatory issues. Am Heart J. 2008;155(3):435–444. - PubMed
-
- Weintraub WS, Jones EL, Craver JM, Guyton RA. Frequency of repeat coronary bypass or coronary angioplasty after coronary artery bypass surgery using saphenous venous grafts. Am J Cardiol. 1994;73(2):103–112. - PubMed
-
- Osswald BR, Blackstone EH, Tochtermann U, et al. Does the completeness of revascularization affect early survival after coronary artery bypass grafting in elderly patients? Eur J Cardiothorac Surg. 2001;20(1):120–125. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

