Synaptic adaptations by alcohol and drugs of abuse: changes in microRNA expression and mRNA regulation
- PMID: 25565954
- PMCID: PMC4267177
- DOI: 10.3389/fnmol.2014.00085
Synaptic adaptations by alcohol and drugs of abuse: changes in microRNA expression and mRNA regulation
Abstract
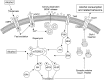
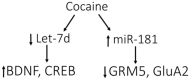
Local translation of mRNAs is a mechanism by which cells can rapidly remodel synaptic structure and function. There is ample evidence for a role of synaptic translation in the neuroadaptations resulting from chronic drug use and abuse. Persistent and coordinated changes of many mRNAs, globally and locally, may have a causal role in complex disorders such as addiction. In this review we examine the evidence that translational regulation by microRNAs drives synaptic remodeling and mRNA expression, which may regulate the transition from recreational to compulsive drug use. microRNAs are small, non-coding RNAs that control the translation of mRNAs in the cell and within spatially restricted sites such as the synapse. microRNAs typically repress the translation of mRNAs into protein by binding to the 3'UTR of their targets. As 'master regulators' of many mRNAs, changes in microRNAs could account for the systemic alterations in mRNA and protein expression observed with drug abuse and dependence. Recent studies indicate that manipulation of microRNAs affects addiction-related behaviors such as the rewarding properties of cocaine, cocaine-seeking behavior, and self-administration rates of alcohol. There is limited evidence, however, regarding how synaptic microRNAs control local mRNA translation during chronic drug exposure and how this contributes to the development of dependence. Here, we discuss research supporting microRNA regulation of local mRNA translation and how drugs of abuse may target this process. The ability of synaptic microRNAs to rapidly regulate mRNAs provides a discrete, localized system that could potentially be used as diagnostic and treatment tools for alcohol and other addiction disorders.
Keywords: cocaine; ethanol; mRNA targets; miRNAs; stimulants; synaptic translation; synaptoneurosomes.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

