Frequency-specific network topologies in the resting human brain
- PMID: 25566037
- PMCID: PMC4273625
- DOI: 10.3389/fnhum.2014.01022
Frequency-specific network topologies in the resting human brain
Abstract
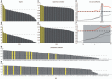
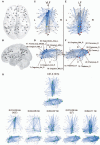
A community is a set of nodes with dense inter-connections, while there are sparse connections between different communities. A hub is a highly connected node with high centrality. It has been shown that both "communities" and "hubs" exist simultaneously in the brain's functional connectivity network (FCN), as estimated by correlations among low-frequency spontaneous fluctuations in functional magnetic resonance imaging (fMRI) signal changes (0.01-0.10 Hz). This indicates that the brain has a spatial organization that promotes both segregation and integration of information. Here, we demonstrate that frequency-specific network topologies that characterize segregation and integration also exist within this frequency range. In investigating the coherence spectrum among 87 brain regions, we found that two frequency bands, 0.01-0.03 Hz (very low frequency [VLF] band) and 0.07-0.09 Hz (low frequency [LF] band), mainly contributed to functional connectivity. Comparing graph theoretical indices for the VLF and LF bands revealed that the network in the former had a higher capacity for information segregation between identified communities than the latter. Hubs in the VLF band were mainly located within the anterior cingulate cortices, whereas those in the LF band were located in the posterior cingulate cortices and thalamus. Thus, depending on the timescale of brain activity, at least two distinct network topologies contributed to information segregation and integration. This suggests that the brain intrinsically has timescale-dependent functional organizations.
Keywords: community; frequency-dependency; functional connectivity; integration; network analysis; resting-state fMRI; rich-club connectivity; segregation.
Figures





References
LinkOut - more resources
Full Text Sources
Other Literature Sources

