Opioid receptor desensitization: mechanisms and its link to tolerance
- PMID: 25566076
- PMCID: PMC4270172
- DOI: 10.3389/fphar.2014.00280
Opioid receptor desensitization: mechanisms and its link to tolerance
Abstract
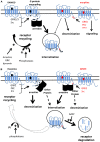
Opioid receptors (OR) are part of the class A of G-protein coupled receptors and the target of the opiates, the most powerful analgesic molecules used in clinic. During a protracted use, a tolerance to analgesic effect develops resulting in a reduction of the effectiveness. So understanding mechanisms of tolerance is a great challenge and may help to find new strategies to tackle this side effect. This review will summarize receptor-related mechanisms that could underlie tolerance especially receptor desensitization. We will focus on the latest data obtained on molecular mechanisms involved in opioid receptor desensitization: phosphorylation, receptor uncoupling, internalization, and post-endocytic fate of the receptor.
Keywords: biased signaling; desensitization; opioid receptors; receptor trafficking; tolerance mechanisms.
Figures

References
-
- Aguila B., Coulbault L., Boulouard M., Leveille F., Davis A., Toth G., et al. (2007). In vitro and in vivo pharmacological profile of UFP-512, a novel selective delta-opioid receptor agonist; correlations between desensitization and tolerance. Br. J. Pharmacol. 152, 1312–1324. 10.1038/sj.bjp.0707497 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

