Presynaptic Mechanisms of l-DOPA-Induced Dyskinesia: The Findings, the Debate, and the Therapeutic Implications
- PMID: 25566170
- PMCID: PMC4266027
- DOI: 10.3389/fneur.2014.00242
Presynaptic Mechanisms of l-DOPA-Induced Dyskinesia: The Findings, the Debate, and the Therapeutic Implications
Abstract
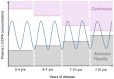
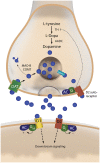
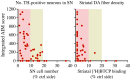
The dopamine (DA) precursor l-DOPA has been the most effective treatment for Parkinson's disease (PD) for over 40 years. However, the response to this treatment changes with disease progression, and most patients develop dyskinesias (abnormal involuntary movements) and motor fluctuations within a few years of l-DOPA therapy. There is wide consensus that these motor complications depend on both pre- and post-synaptic disturbances of nigrostriatal DA transmission. Several presynaptic mechanisms converge to generate large DA swings in the brain concomitant with the peaks-and-troughs of plasma l-DOPA levels, while post-synaptic changes engender abnormal functional responses in dopaminoceptive neurons. While this general picture is well-accepted, the relative contribution of different factors remains a matter of debate. A particularly animated debate has been growing around putative players on the presynaptic side of the cascade. To what extent do presynaptic disturbances in DA transmission depend on deficiency/dysfunction of the DA transporter, aberrant release of DA from serotonin neurons, or gliovascular mechanisms? And does noradrenaline (which is synthetized from DA) play a role? This review article will summarize key findings, controversies, and pending questions regarding the presynaptic mechanisms of l-DOPA-induced dyskinesia. Intriguingly, the debate around these mechanisms has spurred research into previously unexplored facets of brain plasticity that have far-reaching implications to the treatment of neuropsychiatric disease.
Keywords: basal ganglia; dystonia; monoamines; movement disorders; neuropharmacology; neuroplasticity; neuropsychiatry; neurovascular unit.
Figures


References
-
- Leenders KL, Salmon EP, Tyrrell P, Perani D, Brooks DJ, Sager H, et al. The nigrostriatal dopaminergic system assessed in vivo by positron emission tomography in healthy volunteer subjects and patients with Parkinson’s disease. Arch Neurol (1990) 47:1290–8.10.1001/archneur.1990.00530120034007 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

