Staphylococcus aureus biofilms: recent developments in biofilm dispersal
- PMID: 25566513
- PMCID: PMC4275032
- DOI: 10.3389/fcimb.2014.00178
Staphylococcus aureus biofilms: recent developments in biofilm dispersal
Abstract
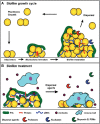
Staphylococcus aureus is a major cause of nosocomial and community-acquired infections and represents a significant burden on the healthcare system. S. aureus attachment to medical implants and host tissue, and the establishment of a mature biofilm, play an important role in the persistence of chronic infections. The formation of a biofilm, and encasement of cells in a polymer-based matrix, decreases the susceptibility to antimicrobials and immune defenses, making these infections difficult to eradicate. During infection, dispersal of cells from the biofilm can result in spread to secondary sites and worsening of the infection. In this review, we discuss the current understanding of the pathways behind biofilm dispersal in S. aureus, with a focus on enzymatic and newly described broad-spectrum dispersal mechanisms. Additionally, we explore potential applications of dispersal in the treatment of biofilm-mediated infections.
Keywords: Staphylococcus aureus; biofilm; dispersal; nuclease; protease; stringent response.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

