Distinct parameters of the basophil activation test reflect the severity and threshold of allergic reactions to peanut
- PMID: 25567046
- PMCID: PMC4282725
- DOI: 10.1016/j.jaci.2014.09.001
Distinct parameters of the basophil activation test reflect the severity and threshold of allergic reactions to peanut
Abstract
Background: The management of peanut allergy relies on allergen avoidance and epinephrine autoinjector for rescue treatment in patients at risk of anaphylaxis. Biomarkers of severity and threshold of allergic reactions to peanut could significantly improve the care for patients with peanut allergy.
Objective: We sought to assess the utility of the basophil activation test (BAT) to predict the severity and threshold of reactivity to peanut during oral food challenges (OFCs).
Methods: The severity of the allergic reaction and the threshold dose during OFCs to peanut were determined. Skin prick tests, measurements of specific IgE to peanut and its components, and BATs to peanut were performed on the day of the challenge.
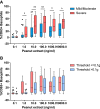
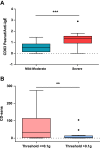
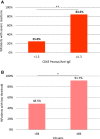
Results: Of the 124 children submitted to OFCs to peanut, 52 (median age, 5 years) reacted with clinical symptoms that ranged from mild oral symptoms to anaphylaxis. Severe reactions occurred in 41% of cases, and 57% reacted to 0.1 g or less of peanut protein. The ratio of the percentage of CD63(+) basophils after stimulation with peanut and after stimulation with anti-IgE (CD63 peanut/anti-IgE) was independently associated with severity (P = .001), whereas the basophil allergen threshold sensitivity CD-sens (1/EC₅₀ × 100, where EC₅₀ is half maximal effective concentration) value was independently associated with the threshold (P = .020) of allergic reactions to peanut during OFCs. Patients with CD63 peanut/anti-IgE levels of 1.3 or greater had an increased risk of severe reactions (relative risk, 3.4; 95% CI, 1.8-6.2). Patients with a CD-sens value of 84 or greater had an increased risk of reacting to 0.1 g or less of peanut protein (relative risk, 1.9; 95% CI, 1.3-2.8).
Conclusions: Basophil reactivity is associated with severity and basophil sensitivity is associated with the threshold of allergic reactions to peanut. CD63 peanut/anti-IgE and CD-sens values can be used to estimate the severity and threshold of allergic reactions during OFCs.
Keywords: Basophil activation test; CD-sens; CD203c; CD63; double-blind; peanut; peanut allergy; placebo-controlled food challenge; sensitivity; severity; threshold.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Development of a tool predicting severity of allergic reaction during peanut challenge.Ann Allergy Asthma Immunol. 2018 Jul;121(1):69-76.e2. doi: 10.1016/j.anai.2018.04.020. Epub 2018 Apr 27. Ann Allergy Asthma Immunol. 2018. PMID: 29709643 Free PMC article. Clinical Trial.
-
Individually dosed omalizumab: an effective treatment for severe peanut allergy.Clin Exp Allergy. 2017 Apr;47(4):540-550. doi: 10.1111/cea.12862. Epub 2017 Jan 10. Clin Exp Allergy. 2017. PMID: 27883239 Clinical Trial.
-
Biomarkers of severity and threshold of allergic reactions during oral peanut challenges.J Allergy Clin Immunol. 2020 Aug;146(2):344-355. doi: 10.1016/j.jaci.2020.03.035. Epub 2020 Apr 18. J Allergy Clin Immunol. 2020. PMID: 32311390 Free PMC article.
-
Basophil Activation Test for the Improved Diagnosis of Peanut and Tree Nut Allergy.Curr Allergy Asthma Rep. 2025 Mar 20;25(1):19. doi: 10.1007/s11882-025-01200-1. Curr Allergy Asthma Rep. 2025. PMID: 40111544 Review.
-
Peanut allergy diagnosis: Moving from basic to more elegant testing.Pediatr Allergy Immunol. 2020 May;31(4):346-357. doi: 10.1111/pai.13215. Epub 2020 Feb 11. Pediatr Allergy Immunol. 2020. PMID: 31945225 Review.
Cited by
-
Early decrease in basophil sensitivity to Ara h 2 precedes sustained unresponsiveness after peanut oral immunotherapy.J Allergy Clin Immunol. 2019 Nov;144(5):1310-1319.e4. doi: 10.1016/j.jaci.2019.07.028. Epub 2019 Aug 1. J Allergy Clin Immunol. 2019. PMID: 31377342 Free PMC article. Clinical Trial.
-
Soy Gly m 8 sIgE Has Limited Value in the Diagnosis of Soy Allergy in Peanut Ara h 2-Sensitized Adults.Int Arch Allergy Immunol. 2023;184(8):767-775. doi: 10.1159/000530026. Epub 2023 Apr 18. Int Arch Allergy Immunol. 2023. PMID: 37071975 Free PMC article.
-
Improving Diagnostic Accuracy in Food Allergy.J Allergy Clin Immunol Pract. 2021 Jan;9(1):71-80. doi: 10.1016/j.jaip.2020.09.037. J Allergy Clin Immunol Pract. 2021. PMID: 33429723 Free PMC article. Review.
-
New Insights in Therapy for Food Allergy.Foods. 2021 May 10;10(5):1037. doi: 10.3390/foods10051037. Foods. 2021. PMID: 34068667 Free PMC article. Review.
-
Allergen Content of Therapeutic Preparations for Allergen-Specific Immunotherapy of European Paper Wasp Venom Allergy.Toxins (Basel). 2022 Apr 15;14(4):284. doi: 10.3390/toxins14040284. Toxins (Basel). 2022. PMID: 35448893 Free PMC article.
References
-
- Sicherer S.H., Munoz-Furlong A., Godbold J.H., Sampson H.A. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–1326. - PubMed
-
- Du Toit G., Katz Y., Sasieni P., Mesher D., Maleki S.J., Fisher H.R. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122:984–991. - PubMed
-
- Lin R.Y., Anderson A.S., Shah S.N., Nurruzzaman F. Increasing anaphylaxis hospitalizations in the first 2 decades of life: New York State, 1990-2006. Ann Allergy Asthma Immunol. 2008;101:387–393. - PubMed
-
- Summers C.W., Pumphrey R.S., Woods C.N., McDowell G., Pemberton P.W., Arkwright P.D. Factors predicting anaphylaxis to peanuts and tree nuts in patients referred to a specialist center. J Allergy Clin Immunol. 2008;121:632–638.e2. - PubMed
-
- Perry T.T., Matsui E.C., Conover-Walker M.K., Wood R.A. Risk of oral food challenges. J Allergy Clin Immunol. 2004;114:1164–1168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

