Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism
- PMID: 25567280
- PMCID: PMC4978465
- DOI: 10.1038/nature13995
Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism
Erratum in
-
Corrigendum: Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism.Nature. 2015 Apr 16;520(7547):388. doi: 10.1038/nature14334. Epub 2015 Mar 4. Nature. 2015. PMID: 25739504 No abstract available.
Abstract
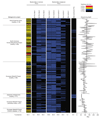
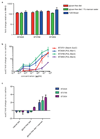
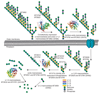
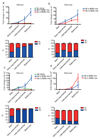
Yeasts, which have been a component of the human diet for at least 7,000 years, possess an elaborate cell wall α-mannan. The influence of yeast mannan on the ecology of the human microbiota is unknown. Here we show that yeast α-mannan is a viable food source for the Gram-negative bacterium Bacteroides thetaiotaomicron, a dominant member of the microbiota. Detailed biochemical analysis and targeted gene disruption studies support a model whereby limited cleavage of α-mannan on the surface generates large oligosaccharides that are subsequently depolymerized to mannose by the action of periplasmic enzymes. Co-culturing studies showed that metabolism of yeast mannan by B. thetaiotaomicron presents a 'selfish' model for the catabolism of this difficult to breakdown polysaccharide. Genomic comparison with B. thetaiotaomicron in conjunction with cell culture studies show that a cohort of highly successful members of the microbiota has evolved to consume sterically-restricted yeast glycans, an adaptation that may reflect the incorporation of eukaryotic microorganisms into the human diet.
Conflict of interest statement
The authors have no competing interests.
Figures
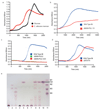
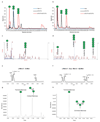

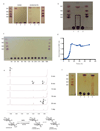

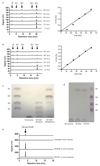




References
-
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. - PubMed
-
- Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–131. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

