Aged iPSCs display an uncommon mitochondrial appearance and fail to undergo in vitro neurogenesis
- PMID: 25567319
- PMCID: PMC4298368
- DOI: 10.18632/aging.100708
Aged iPSCs display an uncommon mitochondrial appearance and fail to undergo in vitro neurogenesis
Abstract
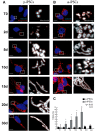
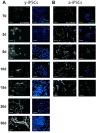
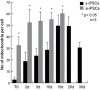
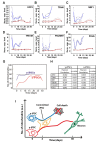
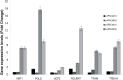
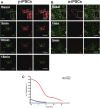
Reprogramming of human fibroblasts into induced pluripotent stem cells (iPSCs) leads to mitochondrial rejuvenation, making iPSCs a candidate model to study the mitochondrial biology during stemness and differentiation. At present, it is generally accepted that iPSCs can be maintained and propagated indefinitely in culture, but no specific studies have addressed this issue. In our study, we investigated features related to the 'biological age' of iPSCs, culturing and analyzing iPSCs kept for prolonged periods in vitro. We have demonstrated that aged iPSCs present an increased number of mitochondria per cell with an altered mitochondrial membrane potential and fail to properly undergo in vitro neurogenesis. In aged iPSCs we have also found an altered expression of genes relevant to mitochondria biogenesis. Overall, our results shed light on the mitochondrial biology of young and aged iPSCs and explore how an altered mitochondrial status may influence neuronal differentiation. Our work suggests to deepen the understanding of the iPSCs biology before considering their use in clinical applications.
Conflict of interest statement
The authors declare no financial, personal or professional competing interests.
Figures

Similar articles
-
Aberrant transcriptional networks in step-wise neurogenesis of paroxysmal kinesigenic dyskinesia-induced pluripotent stem cells.Oncotarget. 2016 Aug 16;7(33):53611-53627. doi: 10.18632/oncotarget.10680. Oncotarget. 2016. PMID: 27449084 Free PMC article.
-
Mitochondrial resetting and metabolic reprogramming in induced pluripotent stem cells and mitochondrial disease modeling.Biochim Biophys Acta. 2016 Apr;1860(4):686-93. doi: 10.1016/j.bbagen.2016.01.009. Epub 2016 Jan 15. Biochim Biophys Acta. 2016. PMID: 26779594 Review.
-
Cytoskeletal dynamics during in vitro neurogenesis of induced pluripotent stem cells (iPSCs).Mol Cell Neurosci. 2016 Dec;77:113-124. doi: 10.1016/j.mcn.2016.10.002. Epub 2016 Oct 15. Mol Cell Neurosci. 2016. PMID: 27756615
-
Revisiting Mitochondrial Function and Metabolism in Pluripotent Stem Cells: Where Do We Stand in Neurological Diseases?Mol Neurobiol. 2017 Apr;54(3):1858-1873. doi: 10.1007/s12035-016-9714-8. Epub 2016 Feb 18. Mol Neurobiol. 2017. PMID: 26892627 Review.
-
Mitochondrial biogenesis and neural differentiation of human iPSC is modulated by idebenone in a developmental stage-dependent manner.Biogerontology. 2017 Aug;18(4):665-677. doi: 10.1007/s10522-017-9718-4. Epub 2017 Jun 22. Biogerontology. 2017. PMID: 28643190 Free PMC article.
Cited by
-
LINCing Senescence and Nuclear Envelope Changes.Cells. 2022 May 30;11(11):1787. doi: 10.3390/cells11111787. Cells. 2022. PMID: 35681483 Free PMC article. Review.
-
Study of mitochondrial respiratory defects on reprogramming to human induced pluripotent stem cells.Aging (Albany NY). 2016 May;8(5):945-57. doi: 10.18632/aging.100950. Aging (Albany NY). 2016. PMID: 27127184 Free PMC article.
-
Systemic Problems: A perspective on stem cell aging and rejuvenation.Aging (Albany NY). 2015 Oct;7(10):754-65. doi: 10.18632/aging.100819. Aging (Albany NY). 2015. PMID: 26540176 Free PMC article. Review.
-
Human iPSCs from Aged Donors Retain Their Mitochondrial Aging Signature.Int J Mol Sci. 2024 Oct 18;25(20):11199. doi: 10.3390/ijms252011199. Int J Mol Sci. 2024. PMID: 39456998 Free PMC article.
-
Human In Vitro Models of Neuroenergetics and Neurometabolic Disturbances: Current Advances and Clinical Perspectives.Stem Cells Transl Med. 2024 Jun 14;13(6):505-514. doi: 10.1093/stcltm/szae021. Stem Cells Transl Med. 2024. PMID: 38588471 Free PMC article. Review.
References
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
-
- Wang A C. Manipulation of Human Induced Pluripotent Stem Cells. Current Protocols in Stem Cell Biology. 2012;23 - PubMed
-
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

