Bone morphogenetic protein 2 gene transduction enhances the osteogenic potential of human urine-derived stem cells
- PMID: 25567327
- PMCID: PMC4417282
- DOI: 10.1186/scrt539
Bone morphogenetic protein 2 gene transduction enhances the osteogenic potential of human urine-derived stem cells
Abstract
Introduction: Urine-derived stem cells (USCs) have the ability to differentiate into osteogenic lineage. Previous studies have raised the possibility that USCs could be used for bone repair. To harness the power of USCs in promoting bone regeneration, methods must be developed to induce USCs to osteogenic lineage efficiently. The present study investigates the effect of lentivirus-encoded bone morphogenetic protein 2 (BMP2) gene transduction on the osteogenic potential of USCs.
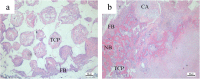
Methods: USCs were isolated from voided urine and transduced with Lentiviral vector encoding BMP2. An in vitro study was performed to detect Lentiviral-BMP2 transduced USCs differentiated towards osteogenic lineage. Furthermore, Lentiviral-BMP2 transduced USCs were transplanted in vivo to examine the ectopic bone formation ability. After six weeks, retrieval samples were obtained for immunostaining and histological analysis.
Results: The results showed that the transduction efficiencies were over 90%, and transduced USCs had high expression levels of the BMP2 gene and secreted BMP2 protein. Alkaline activity and mineral deposition staining demonstrated that transduced USCs differentiate into osteogenic lineages without the addition of osteogenic supplements. Transduced USCs also showed high expression of bone-related markers, including runt-related protein-2 (Runx2) and osteocalcin (OCN), confirming this lentiviral-BMP2 construct provides sufficient stimuli for osteogenic differentiation. Histological analysis indicated that the transduced USCs induced robust new bone formation in nude mice. Six weeks after transplantation, human derived cells were observed to participate in bone formation.
Conclusions: These results demonstrate that BMP2 gene transduction provides an effective method to enhance the osteogenic potential of USCs.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

