Boosted lopinavir- versus boosted atazanavir-containing regimens and immunologic, virologic, and clinical outcomes: a prospective study of HIV-infected individuals in high-income countries
- PMID: 25567330
- PMCID: PMC4447777
- DOI: 10.1093/cid/ciu1167
Boosted lopinavir- versus boosted atazanavir-containing regimens and immunologic, virologic, and clinical outcomes: a prospective study of HIV-infected individuals in high-income countries
Abstract
Background: Current clinical guidelines consider regimens consisting of either ritonavir-boosted atazanavir or ritonavir-boosted lopinavir and a nucleoside reverse transcriptase inhibitor (NRTI) backbone among their recommended and alternative first-line antiretroviral regimens. However, these guidelines are based on limited evidence from randomized clinical trials and clinical experience.
Methods: We compared these regimens with respect to clinical, immunologic, and virologic outcomes using data from prospective studies of human immunodeficiency virus (HIV)-infected individuals in Europe and the United States in the HIV-CAUSAL Collaboration, 2004-2013. Antiretroviral therapy-naive and AIDS-free individuals were followed from the time they started a lopinavir or an atazanavir regimen. We estimated the 'intention-to-treat' effect for atazanavir vs lopinavir regimens on each of the outcomes.
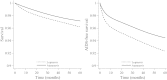
Results: A total of 6668 individuals started a lopinavir regimen (213 deaths, 457 AIDS-defining illnesses or deaths), and 4301 individuals started an atazanavir regimen (83 deaths, 157 AIDS-defining illnesses or deaths). The adjusted intention-to-treat hazard ratios for atazanavir vs lopinavir regimens were 0.70 (95% confidence interval [CI], .53-.91) for death, 0.67 (95% CI, .55-.82) for AIDS-defining illness or death, and 0.91 (95% CI, .84-.99) for virologic failure at 12 months. The mean 12-month increase in CD4 count was 8.15 (95% CI, -.13 to 16.43) cells/µL higher in the atazanavir group. Estimates differed by NRTI backbone.
Conclusions: Our estimates are consistent with a lower mortality, a lower incidence of AIDS-defining illness, a greater 12-month increase in CD4 cell count, and a smaller risk of virologic failure at 12 months for atazanavir compared with lopinavir regimens.
Keywords: HIV; atazanavir; lopinavir; mortality; observational studies.
© The Author 2015. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- European AIDS Clinical Society. EACS guidelines, 2013. Available at: http://www.eacsociety.org/Portals/0/Guidelines_Online_131014.pdf. Accessed 25 November 2013.
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. Department of Health and Human Services, 2014. Available at: http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescent.... Accessed 9 July 2014.
-
- Writing G, Williams I, Churchill D, et al. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2012 (updated November 2013). HIV Med 2014; 15(suppl 1):1–85. - PubMed
-
- Gunthard HF, Aberg JA, Eron JJ, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society–USA Panel. JAMA 2014; 312:410–25. - PubMed
-
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach June 2013, 2013. Available at: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. Accessed 22 August 2014. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials