Resident memory T cells in human health and disease
- PMID: 25568072
- PMCID: PMC4425129
- DOI: 10.1126/scitranslmed.3010641
Resident memory T cells in human health and disease
Abstract
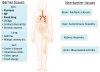
Resident memory T cells are non-recirculating memory T cells that persist long-term in epithelial barrier tissues, including the gastrointestinal tract, lung, skin, and reproductive tract. Resident memory T cells persist in the absence of antigens, have impressive effector functions, and provide rapid on-site immune protection against known pathogens in peripheral tissues. A fundamentally distinct gene expression program differentiates resident memory T cells from circulating T cells. Although these cells likely evolved to provide rapid immune protection against pathogens, autoreactive, aberrantly activated, and malignant resident memory cells contribute to numerous human inflammatory diseases including mycosis fungoides and psoriasis. This review will discuss both the science and medicine of resident memory T cells, exploring how these cells contribute to healthy immune function and discussing what is known about how these cells contribute to human inflammatory and autoimmune diseases.
Copyright © 2015, American Association for the Advancement of Science.
Figures



References
-
- Mackay LK, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nature immunology. 2013 Dec;14:1294. - PubMed
-
- Hogan RJ, et al. Activated antigen-specific CD8+ T cells persist in the lungs following recovery from respiratory virus infections. J Immunol. 2001 Feb 1;166:1813. - PubMed
-
- Wei CH, et al. Tissue-resident memory CD8+ T cells can be deleted by soluble, but not cross-presented antigen. J Immunol. 2005 Nov 15;175:6615. - PubMed
-
- Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001 Mar 23;291:2413. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

