Learning modifies odor mixture processing to improve detection of relevant components
- PMID: 25568113
- PMCID: PMC4287141
- DOI: 10.1523/JNEUROSCI.2345-14.2015
Learning modifies odor mixture processing to improve detection of relevant components
Abstract
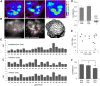
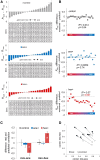
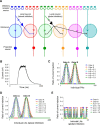
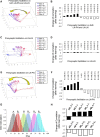
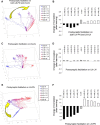
Honey bees have a rich repertoire of olfactory learning behaviors, and they therefore are an excellent model to study plasticity in olfactory circuits. Recent behavioral, physiological, and molecular evidence suggested that the antennal lobe, the first relay of the olfactory system in insects and analog to the olfactory bulb in vertebrates, is involved in associative and nonassociative olfactory learning. Here we use calcium imaging to reveal how responses across antennal lobe projection neurons change after association of an input odor with appetitive reinforcement. After appetitive conditioning to 1-hexanol, the representation of an odor mixture containing 1-hexanol becomes more similar to this odor and less similar to the background odor acetophenone. We then apply computational modeling to investigate how changes in synaptic connectivity can account for the observed plasticity. Our study suggests that experience-dependent modulation of inhibitory interactions in the antennal lobe aids perception of salient odor components mixed with behaviorally irrelevant background odors.
Keywords: antennal lobe; honey bees; olfaction; olfactory learning.
Copyright © 2015 the authors 0270-6474/15/350179-19$15.00/0.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
