Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change
- PMID: 25568120
- PMCID: PMC4287147
- DOI: 10.1523/JNEUROSCI.2310-14.2015
Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change
Abstract
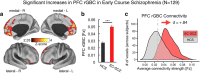
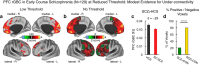
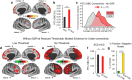
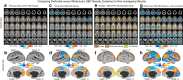
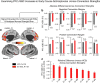
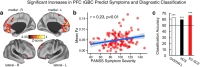
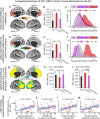
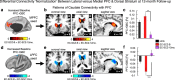
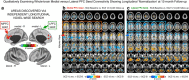
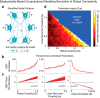
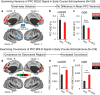
Strong evidence implicates prefrontal cortex (PFC) as a major source of functional impairment in severe mental illness such as schizophrenia. Numerous schizophrenia studies report deficits in PFC structure, activation, and functional connectivity in patients with chronic illness, suggesting that deficient PFC functional connectivity occurs in this disorder. However, the PFC functional connectivity patterns during illness onset and its longitudinal progression remain uncharacterized. Emerging evidence suggests that early-course schizophrenia involves increased PFC glutamate, which might elevate PFC functional connectivity. To test this hypothesis, we examined 129 non-medicated, human subjects diagnosed with early-course schizophrenia and 106 matched healthy human subjects using both whole-brain data-driven and hypothesis-driven PFC analyses of resting-state fMRI. We identified increased PFC connectivity in early-course patients, predictive of symptoms and diagnostic classification, but less evidence for "hypoconnectivity." At the whole-brain level, we observed "hyperconnectivity" around areas centered on the default system, with modest overlap with PFC-specific effects. The PFC hyperconnectivity normalized for a subset of the sample followed longitudinally (n = 25), which also predicted immediate symptom improvement. Biologically informed computational modeling implicates altered overall connection strength in schizophrenia. The initial hyperconnectivity, which may decrease longitudinally, could have prognostic and therapeutic implications.
Keywords: computational modeling; first episode; hyperconnectivity; longitudinal; prefrontal cortex; schizophrenia.
Copyright © 2015 the authors 0270-6474/15/350267-20$15.00/0.
Figures


References
-
- Anticevic A, Brumbaugh MS, Winkler AM, Lombardo LE, Barrett J, Corlett PR, Kober H, Gruber J, Repovs G, Cole MW, Krystal JH, Pearlson GD, Glahn DC. Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry. 2013;73:565–573. doi: 10.1016/j.biopsych.2012.07.031. - DOI - PMC - PubMed
-
- Anticevic A, Gancsos M, Murray JD, Repovs G, Driesen NR, Ennis DJ, Niciu MJ, Morgan PT, Surti TS, Bloch MH, Ramani R, Smith M, Wang XJ, Krystal JH, Corlett PR. NMDA receptor function in large-scale anti-correlated neural systems with implications for cognition and schizophrenia. Proc Natl Acad Sci U S A. 2012b;109:16720–16725. doi: 10.1073/pnas.1208494109. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
