An angiotensin-(1-7) peptidase in the kidney cortex, proximal tubules, and human HK-2 epithelial cells that is distinct from insulin-degrading enzyme
- PMID: 25568136
- PMCID: PMC4360035
- DOI: 10.1152/ajprenal.00609.2014
An angiotensin-(1-7) peptidase in the kidney cortex, proximal tubules, and human HK-2 epithelial cells that is distinct from insulin-degrading enzyme
Abstract
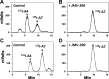
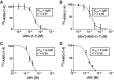
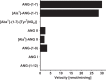
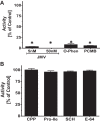
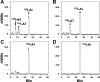
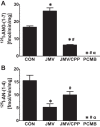
Angiotensin 1-7 [ANG-(1-7)] is expressed within the kidney and exhibits renoprotective actions that antagonize the inflammatory, fibrotic, and pro-oxidant effects of ANG II. We previously identified an peptidase that preferentially metabolized ANG-(1-7) to ANG-(1-4) in the brain medulla and cerebrospinal fluid (CSF) of sheep (Marshall AC, Pirro NT, Rose JC, Diz DI, Chappell MC. J Neurochem 130: 313-323, 2014); thus the present study established the expression of the peptidase in the kidney. Utilizing a sensitive HPLC-based approach, we demonstrate a peptidase activity that hydrolyzed ANG-(1-7) to ANG-(1-4) in the sheep cortex, isolated tubules, and human HK-2 renal epithelial cells. The peptidase was markedly sensitive to the metallopeptidase inhibitor JMV-390; human HK-2 cells expressed subnanomolar sensitivity (IC50 = 0.5 nM) and the highest specific activity (123 ± 5 fmol·min(-1)·mg(-1)) compared with the tubules (96 ± 12 fmol·min(-1)·mg(-1)) and cortex (107 ± 9 fmol·min(-1)·mg(-1)). The peptidase was purified 41-fold from HK-2 cells; the activity was sensitive to JMV-390, the chelator o-phenanthroline, and the mercury-containing compound p-chloromercuribenzoic acid (PCMB), but not to selective inhibitors against neprilysin, neurolysin and thimet oligopeptidase. Both ANG-(1-7) and its endogenous analog [Ala(1)]-ANG-(1-7) (alamandine) were preferentially hydrolyzed by the peptidase compared with ANG II, [Asp(1)]-ANG II, ANG I, and ANG-(1-12). Although the ANG-(1-7) peptidase and insulin-degrading enzyme (IDE) share similar inhibitor characteristics of a metallothiolendopeptidase, we demonstrate marked differences in substrate specificity, which suggest these peptidases are distinct. We conclude that an ANG-(1-7) peptidase is expressed within the renal proximal tubule and may play a potential role in the renal renin-angiotensin system to regulate ANG-(1-7) tone.
Keywords: ANG-(1–7); HK-2 epithelial cells; endopeptidase; proximal tubules.
Copyright © 2015 the American Physiological Society.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

