Contribution of MxB oligomerization to HIV-1 capsid binding and restriction
- PMID: 25568212
- PMCID: PMC4337540
- DOI: 10.1128/JVI.03730-14
Contribution of MxB oligomerization to HIV-1 capsid binding and restriction
Abstract
The alpha interferon (IFN-α)-inducible restriction factor myxovirus B (MxB) blocks HIV-1 infection after reverse transcription but prior to integration. MxB binds to the HIV-1 core, which is composed of capsid protein, and this interaction leads to inhibition of the uncoating process of HIV-1. Previous studies suggested that HIV-1 restriction by MxB requires binding to capsid. This work tests the hypothesis that MxB oligomerization is important for the ability of MxB to bind to the HIV-1 core. For this purpose, we modeled the structure of MxB using the published tertiary structure of MxA. The modeled structure of MxB guided our mutagenic studies and led to the discovery of several MxB variants that lose the capacity to oligomerize. In agreement with our hypothesis, MxB variants that lost the oligomerization capacity also lost the ability to bind to the HIV-1 core. MxB variants deficient for oligomerization were not able to block HIV-1 infection. Overall, our work showed that oligomerization is required for the ability of MxB to bind to the HIV-1 core and block HIV-1 infection.
Importance: MxB is a novel restriction factor that blocks infection of HIV-1. MxB is inducible by IFN-α, particularly in T cells. The current work studies the oligomerization determinants of MxB and carefully explores the contribution of oligomerization to capsid binding and restriction. This work takes advantage of the current structure of MxA and models the structure of MxB, which is used to guide structure-function studies. This work leads to the conclusion that MxB oligomerization is important for HIV-1 capsid binding and restriction.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



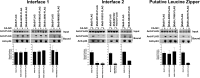

References
-
- Melén K, Keskinen P, Ronni T, Sareneva T, Lounatmaa K, Julkunen I. 1996. Human MxB protein, an interferon-alpha-inducible GTPase, contains a nuclear targeting signal and is localized in the heterochromatin region beneath the nuclear envelope. J Biol Chem 271:23478–23486. doi:10.1074/jbc.271.38.23478. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

