Molecules Great and Small: The Complement System
- PMID: 25568220
- PMCID: PMC4559511
- DOI: 10.2215/CJN.06230614
Molecules Great and Small: The Complement System
Abstract
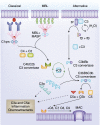
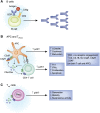
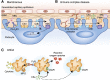
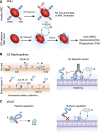
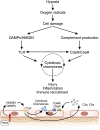
The complement cascade, traditionally considered an effector arm of innate immunity required for host defense against pathogens, is now recognized as a crucial pathogenic mediator of various kidney diseases. Complement components produced by the liver and circulating in the plasma undergo activation through the classical and/or mannose-binding lectin pathways to mediate anti-HLA antibody-initiated kidney transplant rejection and autoantibody-initiated GN, the latter including membranous glomerulopathy, antiglomerular basement membrane disease, and lupus nephritis. Inherited and/or acquired abnormalities of complement regulators, which requisitely limit restraint on alternative pathway complement activation, contribute to the pathogenesis of the C3 nephropathies and atypical hemolytic uremic syndrome. Increasing evidence links complement produced by endothelial cells and/or tubular cells to the pathogenesis of kidney ischemia-reperfusion injury and progressive kidney fibrosis. Data emerging since the mid-2000s additionally show that immune cells, including T cells and antigen-presenting cells, produce alternative pathway complement components during cognate interactions. The subsequent local complement activation yields production of the anaphylatoxins C3a and C5a, which bind to their respective receptors (C3aR and C5aR) on both partners to augment effector T-cell proliferation and survival, while simultaneously inhibiting regulatory T-cell induction and function. This immune cell-derived complement enhances pathogenic alloreactive T-cell immunity that results in transplant rejection and likely contributes to the pathogenesis of other T cell-mediated kidney diseases. C5a/C5aR ligations on neutrophils have additionally been shown to contribute to vascular inflammation in models of ANCA-mediated renal vasculitis. New translational immunology efforts along with the development of pharmacologic agents that block human complement components and receptors now permit testing of the intriguing concept that targeting complement in patients with an assortment of kidney diseases has the potential to abrogate disease progression and improve patient health.
Keywords: GN; complement; immunology; transplantation.
Copyright © 2015 by the American Society of Nephrology.
Figures


References
-
- Goodrum KJ: Complement component C3 secretion by mouse macrophage-like cell lines. J Leukoc Biol 41: 295–301, 1987 - PubMed
-
- Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, Voorhorst M, Ugurlar D, Rosati S, Heck AJ, van de Winkel JG, Wilson IA, Koster AJ, Taylor RP, Saphire EO, Burton DR, Schuurman J, Gros P, Parren PW: Complement is activated by IgG hexamers assembled at the cell surface. Science 343: 1260–1263, 2014 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

