Rare mutation in the SLC26A3 transporter causes life-long diarrhoea with metabolic alkalosis
- PMID: 25568271
- PMCID: PMC4289764
- DOI: 10.1136/bcr-2014-206849
Rare mutation in the SLC26A3 transporter causes life-long diarrhoea with metabolic alkalosis
Abstract
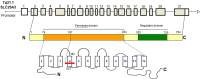
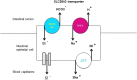
SLC26A3, a chloride/bicarbonate transporter mainly expressed in the intestines, plays a pivotal role in chloride absorption. We present a 23-year-old woman with a history of congenital chloride diarrhoea (CCD) and renal transplant who was admitted for rehydration and treatment of acute kidney injury after she presented with an acute diarrhoeal episode. Laboratory investigations confirmed metabolic alkalosis and severe hypochloraemia, consistent with her underlying CCD. This contrasts with most other forms of diarrhoea, which are normally associated with metabolic acidosis. Genetic testing was offered and revealed a homozygous non-sense mutation in SLC26A3 (Gly-187-Stop). This loss-of-function mutation results in bicarbonate retention in the blood and chloride loss into the intestinal lumen. Symptomatic management with daily NaCl and KCl oral syrups was supplemented with omeprazole therapy. The loss of her own kidneys is most likely due to crystal-induced nephropathy secondary to chronic volume contraction and chloride depletion. This case summarises the pathophysiology and management of CCD.
2015 BMJ Publishing Group Ltd.
Figures
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
