DEPTOR is linked to a TORC1-p21 survival proliferation pathway in multiple myeloma cells
- PMID: 25568666
- PMCID: PMC4279438
- DOI: 10.18632/genesandcancer.44
DEPTOR is linked to a TORC1-p21 survival proliferation pathway in multiple myeloma cells
Abstract
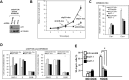
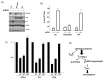
We investigated the mechanism by which gene silencing of the mTOR inhibitor, DEPTOR, induces cytoreductive effects on multiple myeloma (MM) cells. DEPTOR knockdown resulted in anti-MM effects in several MM cell lines. Using an inducible shRNA to silence DEPTOR, 8226 MM cells underwent TORC1 activation, downregulation of AKT/SGK activity, apoptosis, cell cycle arrest and senescence. These latter cytotoxic effects were prevented by TORC1 paralysis (Raptor knockdown) but not by over-expression of AKT activity. In addition, DEPTOR knockdown-induced MM death was not associated with activation of the unfolded protein response, suggesting that enhanced ER stress did not play a role. In contrast, DEPTOR knockdown in 8226 cells induced p21 expression, independent of p53, and p21 knockdown prevented all of the cytotoxic effects following DEPTOR silencing. DEPTOR silencing resulted in p21 upregulation in additional MM cell lines. Furthermore, DEPTOR silencing in a murine xenograft model resulted in anti-MM effects associated with p21 upregulation. DEPTOR knockdown also resulted in a decreased expression of p21-targeting miRNAs and transfection of miRNA mimics prevented p21 upregulation and apoptosis following DEPTOR silencing. Use of a shRNA-resistant DEPTOR construct ruled out off-target effects of the shRNA. These results indicate that DEPTOR regulates growth and survival of MM cells via a TORC1/p21 pathway and suggest an involvement of p21-targeted miRNAs.
Keywords: AKT; DEPTOR; ER stress; Multiple Myeloma; mTORC1; p21.
Figures






References
-
- Boyd KD, Walker BA, Wardell CP, Ross FM, Gregory WM, Davies FE, et al. High expression levels of the mTOR inhibitor DEPTOR are predictive of response to thalidomide in myeloma. Leuk & Lymphoma. 2010;51:2126–2129. - PubMed
-
- Carrasco DR, Tonon G, Huang Y, Zhang Y, Sinha R, Feng B, et al. High resolution genomic profiles define distinct clinicl-pathogenetic subgroups of multiple myeloma patients. Cancer Cell. 2006;9:313–325. - PubMed
-
- Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, et al. Activation of AKT and eIF4E survival pathways by rapamycin-mediated mTOR inhibition. Cancer Res. 2005;65:7052–58. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

