Recent advances in the development of anti-HER2 antibodies and antibody-drug conjugates
- PMID: 25568875
- PMCID: PMC4260046
- DOI: 10.3978/j.issn.2305-5839.2014.08.13
Recent advances in the development of anti-HER2 antibodies and antibody-drug conjugates
Abstract
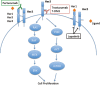
Human epidermal growth factor receptor 2 (HER2)-targeted therapies have revolutionized the treatment of HER2-positive breast cancer, both in the metastatic and early stage settings. While trastuzumab and lapatinib had been the mainstays of treatment in combination with chemotherapy, innate and acquired resistance to these therapies occur. More recently, two additional HER2-directed therapies have been approved for HER2-positive breast cancer. Pertuzumab is a humanized monoclonal antibody that binds to the extracellular portion of the receptor on a domain distinct from the binding site of trastuzumab. The addition of pertuzumab to trastuzumab results in synergistic tumor cell inhibition and has been shown to significantly improve clinical outcomes for patients with HER2-positive metastatic breast cancer (MBC) compared to trastuzumab plus chemotherapy alone. In addition, ado-trastuzumab emtansine (T-DM1), a novel antibody-drug conjugate linking trastuzumab with the cytotoxic maytansinoid, DM1, is an effective treatment for HER2-positive breast cancer that has progressed on other HER2-directed therapies. Both pertuzumab and T-DM1 are relatively well tolerated. This review presents the mechanisms of action as well as phase I, II and III clinical data describing the safety and efficacy of pertuzumab and T-DM1 for HER2-positive breast cancer.
Keywords: Human epidermal growth factor receptor 2 (HER2); T-DM1; antibody-drug conjugate; breast cancer; pertuzumab.
Figures
References
-
- Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989;244:707-12. - PubMed
-
- Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997-4013. - PubMed
-
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001;2:127-37. - PubMed
-
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82. - PubMed
-
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
