Quantifying and monitoring overdiagnosis in cancer screening: a systematic review of methods
- PMID: 25569206
- PMCID: PMC4332263
- DOI: 10.1136/bmj.g7773
Quantifying and monitoring overdiagnosis in cancer screening: a systematic review of methods
Abstract
Objective: To determine the optimal method for quantifying and monitoring overdiagnosis in cancer screening over time.
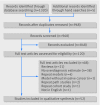
Design: Systematic review of primary research studies of any design that quantified overdiagnosis from screening for nine types of cancer. We used explicit criteria to critically appraise individual studies and assess strength of the body of evidence for each study design (double blinded review), and assessed the potential for each study design to accurately quantify and monitor overdiagnosis over time.
Data sources: PubMed and Embase up to 28 February 2014; hand searching of systematic reviews.
Eligibility criteria for selecting studies: English language studies of any design that quantified overdiagnosis for any of nine common cancers (prostate, breast, lung, colorectal, melanoma, bladder, renal, thyroid, and uterine); excluded case series, case reports, and reviews that only reported results of other studies.
Results: 52 studies met the inclusion criteria. We grouped studies into four methodological categories: (1) follow-up of a well designed randomized controlled trial (n=3), which has low risk of bias but may not be generalizable and is not suitable for monitoring; (2) pathological or imaging studies (n=8), drawing conclusions about overdiagnosis by examining biological characteristics of cancers, a simple design limited by the uncertain assumption that the measured characteristics are highly correlated with disease progression; (3) modeling studies (n=21), which can be done in a shorter time frame but require complex mathematical equations simulating the natural course of screen detected cancer, the fundamental unknown question; and (4) ecological and cohort studies (n=20), which are suitable for monitoring over time but are limited by a lack of agreed standards, by variable data quality, by inadequate follow-up time, and by the potential for population level confounders. Some ecological and cohort studies, however, have addressed these potential weaknesses in reasonable ways.
Conclusions: Well conducted ecological and cohort studies in multiple settings are the most appropriate approach for quantifying and monitoring overdiagnosis in cancer screening programs. To support this work, we need internationally agreed standards for ecological and cohort studies and a multinational team of unbiased researchers to perform ongoing analysis.
© Carter et al 2015.
Conflict of interest statement
Competing interests: All authors have completed the Unified Competing Interest form at
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous