Cell lineage analysis in human brain using endogenous retroelements
- PMID: 25569347
- PMCID: PMC4299461
- DOI: 10.1016/j.neuron.2014.12.028
Cell lineage analysis in human brain using endogenous retroelements
Abstract
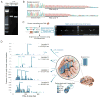
Somatic mutations occur during brain development and are increasingly implicated as a cause of neurogenetic disease. However, the patterns in which somatic mutations distribute in the human brain are unknown. We used high-coverage whole-genome sequencing of single neurons from a normal individual to identify spontaneous somatic mutations as clonal marks to track cell lineages in human brain. Somatic mutation analyses in >30 locations throughout the nervous system identified multiple lineages and sublineages of cells marked by different LINE-1 (L1) retrotransposition events and subsequent mutation of poly-A microsatellites within L1. One clone contained thousands of cells limited to the left middle frontal gyrus, whereas a second distinct clone contained millions of cells distributed over the entire left hemisphere. These patterns mirror known somatic mutation disorders of brain development and suggest that focally distributed mutations are also prevalent in normal brains. Single-cell analysis of somatic mutation enables tracing of cell lineage clones in human brain.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures



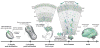
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

