Different infectivity of HIV-1 strains is linked to number of envelope trimers required for entry
- PMID: 25569556
- PMCID: PMC4287578
- DOI: 10.1371/journal.ppat.1004595
Different infectivity of HIV-1 strains is linked to number of envelope trimers required for entry
Abstract
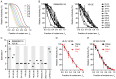
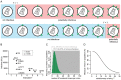
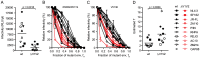
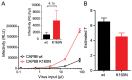
HIV-1 enters target cells by virtue of envelope glycoprotein trimers that are incorporated at low density in the viral membrane. How many trimers are required to interact with target cell receptors to mediate virus entry, the HIV entry stoichiometry, still awaits clarification. Here, we provide estimates of the HIV entry stoichiometry utilizing a combined approach of experimental analyses and mathematical modeling. We demonstrate that divergent HIV strains differ in their stoichiometry of entry and require between 1 to 7 trimers, with most strains depending on 2 to 3 trimers to complete infection. Envelope modifications that perturb trimer structure lead to an increase in the entry stoichiometry, as did naturally occurring antibody or entry inhibitor escape mutations. Highlighting the physiological relevance of our findings, a high entry stoichiometry correlated with low virus infectivity and slow virus entry kinetics. The entry stoichiometry therefore directly influences HIV transmission, as trimer number requirements will dictate the infectivity of virus populations and efficacy of neutralizing antibodies. Thereby our results render consideration of stoichiometric concepts relevant for developing antibody-based vaccines and therapeutics against HIV.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Wilen CB, Tilton JC, Doms RW (2012) Molecular mechanisms of HIV entry. Adv Exp Med Biol 726: 223–242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

