Molecular determinants of positive allosteric modulation of the human metabotropic glutamate receptor 2
- PMID: 25571949
- PMCID: PMC4403101
- DOI: 10.1111/bph.13065
Molecular determinants of positive allosteric modulation of the human metabotropic glutamate receptor 2
Abstract
Background and purpose: The activation of the metabotropic glutamate receptor 2 (mGlu2 ) reduces glutamatergic transmission in brain regions where excess excitatory signalling is implicated in disorders such as anxiety and schizophrenia. Positive allosteric modulators (PAMs) can provide a fine-tuned potentiation of these receptors' function and are being investigated as a novel therapeutic approach. An extensive set of mutant human mGlu2 receptors were used to investigate the molecular determinants that are important for positive allosteric modulation at this receptor.
Experimental approach: Site-directed mutagenesis, binding and functional assays were employed to identify amino acids important for the activity of nine PAMs. The data from the radioligand binding and mutagenesis studies were used with computational docking to predict a binding mode at an mGlu2 receptor model based on the recent structure of the mGlu1 receptor.
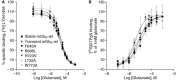
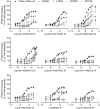
Key results: New amino acids in TM3 (R635, L639, F643), TM5 (L732) and TM6 (W773, F776) were identified for the first time as playing an important role in the activity of mGlu2 PAMs.
Conclusions and implications: This extensive study furthers our understanding of positive allosteric modulation of the mGlu2 receptor and can contribute to improved future design of mGlu2 PAMs.
© 2015 The British Pharmacological Society.
Figures



Similar articles
-
Differential Pharmacology and Binding of mGlu2 Receptor Allosteric Modulators.Mol Pharmacol. 2018 May;93(5):526-540. doi: 10.1124/mol.117.110114. Epub 2018 Mar 15. Mol Pharmacol. 2018. PMID: 29545267 Free PMC article.
-
Pharmacological and molecular characterization of the positive allosteric modulators of metabotropic glutamate receptor 2.Neuropharmacology. 2016 Dec;111:253-265. doi: 10.1016/j.neuropharm.2016.08.032. Epub 2016 Aug 30. Neuropharmacology. 2016. PMID: 27590915 Review.
-
Pharmacological characterization of JNJ-40068782, a new potent, selective, and systemically active positive allosteric modulator of the mGlu2 receptor and its radioligand [3H]JNJ-40068782.J Pharmacol Exp Ther. 2013 Sep;346(3):514-27. doi: 10.1124/jpet.113.204990. Epub 2013 Jun 13. J Pharmacol Exp Ther. 2013. PMID: 23766542
-
Co-operative binding assay for the characterization of mGlu4 allosteric modulators.Neuropharmacology. 2015 Oct;97:142-8. doi: 10.1016/j.neuropharm.2015.05.017. Epub 2015 May 27. Neuropharmacology. 2015. PMID: 26025660 Free PMC article.
-
Reprint of Pharmacological and molecular characterization of the positive allosteric modulators of metabotropic glutamate receptor 2.Neuropharmacology. 2017 Mar 15;115:115-127. doi: 10.1016/j.neuropharm.2016.08.040. Epub 2017 Feb 16. Neuropharmacology. 2017. PMID: 28216000 Review.
Cited by
-
Positive allosteric modulators of metabotropic glutamate 2 receptors in schizophrenia treatment.Trends Neurosci. 2015 Aug;38(8):506-16. doi: 10.1016/j.tins.2015.06.002. Epub 2015 Jul 4. Trends Neurosci. 2015. PMID: 26148747 Free PMC article. Review.
-
Selective Photoswitchable Allosteric Agonist of a G Protein-Coupled Receptor.J Am Chem Soc. 2021 Jun 23;143(24):8951-8956. doi: 10.1021/jacs.1c02586. Epub 2021 Jun 11. J Am Chem Soc. 2021. PMID: 34115935 Free PMC article.
-
Allosteric ligands control the activation of a class C GPCR heterodimer by acting at the transmembrane interface.Elife. 2021 Dec 6;10:e70188. doi: 10.7554/eLife.70188. Elife. 2021. PMID: 34866572 Free PMC article.
-
Molecular mechanism of positive allosteric modulation of the metabotropic glutamate receptor 2 by JNJ-46281222.Br J Pharmacol. 2016 Feb;173(3):588-600. doi: 10.1111/bph.13390. Epub 2016 Jan 13. Br J Pharmacol. 2016. PMID: 26589404 Free PMC article.
-
Differential Pharmacology and Binding of mGlu2 Receptor Allosteric Modulators.Mol Pharmacol. 2018 May;93(5):526-540. doi: 10.1124/mol.117.110114. Epub 2018 Mar 15. Mol Pharmacol. 2018. PMID: 29545267 Free PMC article.
References
-
- Bonnefous C, Vernier J-M, Hutchinson JH, Gardner MF, Cramer M, James JK, et al. Biphenyl-indanones: allosteric potentiators of the metabotropic glutamate subtype 2 receptor. Bioorg Med Chem Lett. 2005;15:4354–4358. - PubMed
-
- Bruno A, Guadix AE, Costantino G. Molecular dynamics simulation of the heterodimeric mGluR2/5HT2A complex. An atomistic resolution study of a potential new target in psychiatric conditions. J Chem Inf Model. 2009;49:1602–1616. - PubMed
-
- Chiechio S, Nicoletti F. Metabotropic glutamate receptors and the control of chronic pain. Curr Opin Pharmacol. 2012;12:28–34. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

