Biological targets and mechanisms of action of natural products from marine cyanobacteria
- PMID: 25571978
- PMCID: PMC4344435
- DOI: 10.1039/c4np00104d
Biological targets and mechanisms of action of natural products from marine cyanobacteria
Abstract
Marine cyanobacteria are an ancient group of organisms and prolific producers of bioactive secondary metabolites. These compounds are presumably optimized by evolution over billions of years to exert high affinity for their intended biological target in the ecologically relevant organism but likely also possess activity in different biological contexts such as human cells. Screening of marine cyanobacterial extracts for bioactive natural products has largely focused on cancer cell viability; however, diversification of the screening platform led to the characterization of many new bioactive compounds. Targets of compounds have oftentimes been elusive if the compounds were discovered through phenotypic assays. Over the past few years, technology has advanced to determine mechanism of action (MOA) and targets through reverse chemical genetic and proteomic approaches, which has been applied to certain cyanobacterial compounds and will be discussed in this review. Some cyanobacterial molecules are the most-potent-in-class inhibitors and therefore may become valuable tools for chemical biology to probe protein function but also be templates for novel drugs, assuming in vitro potency translates into cellular and in vivo activity. Our review will focus on compounds for which the direct targets have been deciphered or which were found to target a novel pathway, and link them to disease states where target modulation may be beneficial.
Conflict of interest statement
Conflict of Interest
HL is a co-founder of Oceanyx Pharmaceuticals, Inc., which has been licensing various patents and patent applications related to cyanobacterial compounds discussed in this review.
Figures
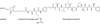



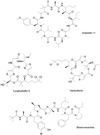
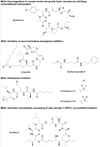
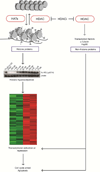
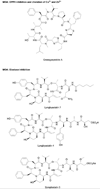


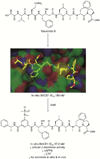
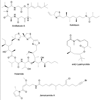
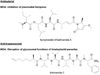
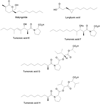
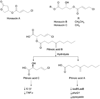
References
-
- Cong F, Cheung AK, Huang SA. Annu. Rev. Pharmacol. Toxicol. 2012;52:57–78. - PubMed
-
- Hurco O. Neurochem. Int. 2012;61:892–898. - PubMed
-
- Ziegler S, Pries V, Hedberg C, Waldmann H. Angew. Chem. Int. Ed. 2013;52:2744–2794. - PubMed
-
- Breinbauer R, Vetter IR, Waldmann H. Angew. Chem. Int. Ed. 2002;41:2878–2890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

