JAK-STAT pathway activation in malignant and nonmalignant cells contributes to MPN pathogenesis and therapeutic response
- PMID: 25572172
- PMCID: PMC4355105
- DOI: 10.1158/2159-8290.CD-14-0736
JAK-STAT pathway activation in malignant and nonmalignant cells contributes to MPN pathogenesis and therapeutic response
Abstract
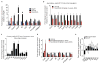
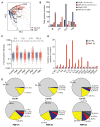
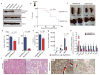
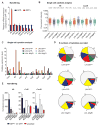
The identification of JAK2/MPL mutations in patients with myeloproliferative neoplasms (MPN) has led to the clinical development of JAK kinase inhibitors, including ruxolitinib. Ruxolitinib reduces splenomegaly and systemic symptoms in myelofibrosis and improves overall survival; however, the mechanism by which JAK inhibitors achieve efficacy has not been delineated. Patients with MPN present with increased levels of circulating proinflammatory cytokines, which are mitigated by JAK inhibitor therapy. We sought to elucidate mechanisms by which JAK inhibitors attenuate cytokine-mediated pathophysiology. Single-cell profiling demonstrated that hematopoietic cells from myelofibrosis models and patient samples aberrantly secrete inflammatory cytokines. Pan-hematopoietic Stat3 deletion reduced disease severity and attenuated cytokine secretion, with similar efficacy as observed with ruxolitinib therapy. In contrast, Stat3 deletion restricted to MPN cells did not reduce disease severity or cytokine production. Consistent with these observations, we found that malignant and nonmalignant cells aberrantly secrete cytokines and JAK inhibition reduces cytokine production from both populations.
Significance: Our results demonstrate that JAK-STAT3-mediated cytokine production from malignant and nonmalignant cells contributes to MPN pathogenesis and that JAK inhibition in both populations is required for therapeutic efficacy. These findings provide novel insight into the mechanisms by which JAK kinase inhibition achieves therapeutic efficacy in MPNs.
©2015 American Association for Cancer Research.
Conflict of interest statement
Conflict of interest disclosure statement: TR, MM, MR, and VR are employees of Novartis Institutes for BioMedical Research. PK is currently an employee of Amgen and JSF was an employee of Incyte Corporation. RLL received grant support from Novartis for this study.
Figures


Comment in
-
Aberrant cytokine production by nonmalignant cells in the pathogenesis of myeloproliferative tumors and response to JAK inhibitor therapies.Cancer Discov. 2015 Mar;5(3):234-6. doi: 10.1158/2159-8290.CD-15-0095. Cancer Discov. 2015. PMID: 25749974 Free PMC article.
References
-
- Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361:1872–85. - PubMed
-
- Delhommeau F, Jeziorowska D, Marzac C, Casadevall N. Molecular aspects of myeloproliferative neoplasms. Int J Hematol. 2010;91:165–73. - PubMed
-
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–61. - PubMed
-
- Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. The New England journal of medicine. 2005;352:1779–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 CA143798/CA/NCI NIH HHS/United States
- T32GM007739/GM/NIGMS NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U01 DK104331/DK/NIDDK NIH HHS/United States
- 1R01CA138234-01/CA/NCI NIH HHS/United States
- 1U01DK104331-01/DK/NIDDK NIH HHS/United States
- R01 CA151949/CA/NCI NIH HHS/United States
- 1R01CA151949-01/CA/NCI NIH HHS/United States
- T32 GM007739/GM/NIGMS NIH HHS/United States
- R01 CA138234/CA/NCI NIH HHS/United States
- U54CA143798-01/CA/NCI NIH HHS/United States
- F30 CA183497/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

