Circadian rhythms of crawling and swimming in the nudibranch mollusc Melibe leonina
- PMID: 25572214
- PMCID: PMC4479187
- DOI: 10.1086/BBLv227n3p263
Circadian rhythms of crawling and swimming in the nudibranch mollusc Melibe leonina
Abstract
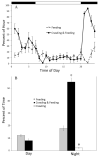
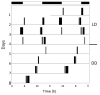
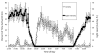
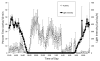
Daily rhythms of activity driven by circadian clocks are expressed by many organisms, including molluscs. We initiated this study, with the nudibranch Melibe leonina, with four goals in mind: (1) determine which behaviors are expressed with a daily rhythm; (2) investigate which of these rhythmic behaviors are controlled by a circadian clock; (3) determine if a circadian clock is associated with the eyes or optic ganglia of Melibe, as it is in several other gastropods; and (4) test the hypothesis that Melibe can use extraocular photoreceptors to synchronize its daily rhythms to natural light-dark cycles. To address these goals, we analyzed the behavior of 55 animals exposed to either artificial or natural light-dark cycles, followed by constant darkness. We also repeated this experiment using 10 animals that had their eyes removed. Individuals did not express daily rhythms of feeding, but they swam and crawled more at night. This pattern of locomotion persisted in constant darkness, indicating the presence of a circadian clock. Eyeless animals also expressed a daily rhythm of locomotion, with more locomotion at night. The fact that eyeless animals synchronized their locomotion to the light-dark cycle suggests that they can detect light using extraocular photoreceptors. However, in constant darkness, these rhythms deteriorated, suggesting that the clock neurons that influence locomotion may be located in, or near, the eyes. Thus, locomotion in Melibe appears to be influenced by both ocular and extraocular photoreceptors, although the former appear to have a greater influence on the expression of circadian rhythms.
© 2014 Marine Biological Laboratory.
Figures



Similar articles
-
Localization and expression of putative circadian clock transcripts in the brain of the nudibranch Melibe leonina.Comp Biochem Physiol A Mol Integr Physiol. 2018 Sep;223:52-59. doi: 10.1016/j.cbpa.2018.05.002. Epub 2018 May 9. Comp Biochem Physiol A Mol Integr Physiol. 2018. PMID: 29753034 Free PMC article.
-
Serotonin influences locomotion in the nudibranch mollusc Melibe leonina.Biol Bull. 2011 Jun;220(3):155-60. doi: 10.1086/BBLv220n3p155. Biol Bull. 2011. PMID: 21712224 Free PMC article.
-
Light and feeding entrainment of the molecular circadian clock in a marine teleost (Sparus aurata).Chronobiol Int. 2013 Jun;30(5):649-61. doi: 10.3109/07420528.2013.775143. Epub 2013 May 20. Chronobiol Int. 2013. PMID: 23688119
-
Natural environmental cues and circadian rhythms of behaviour--a perspective.Chronobiol Int. 1986;3(4):247-53. doi: 10.3109/07420528609079542. Chronobiol Int. 1986. PMID: 3315254 Review.
-
How cells tell time.Trends Cell Biol. 1998 Jun;8(6):224-30. doi: 10.1016/s0962-8924(98)01269-0. Trends Cell Biol. 1998. PMID: 9695846 Review.
Cited by
-
Sequences of Circadian Clock Proteins in the Nudibranch Molluscs Hermissenda crassicornis, Melibe leonina, and Tritonia diomedea.Biol Bull. 2018 Jun;234(3):207-218. doi: 10.1086/698467. Epub 2018 Jun 5. Biol Bull. 2018. PMID: 29949437 Free PMC article.
-
The Digestive Diverticula in the Carnivorous Nudibranch, Melibe leonina, Do Not Contain Photosynthetic Symbionts.Integr Org Biol. 2021 May 21;3(1):obab015. doi: 10.1093/iob/obab015. eCollection 2021. Integr Org Biol. 2021. PMID: 34337322 Free PMC article.
-
Neural mechanism of circadian clock-based photoperiodism in insects and snails.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024 Jul;210(4):601-625. doi: 10.1007/s00359-023-01662-6. Epub 2023 Aug 18. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024. PMID: 37596422 Free PMC article. Review.
-
Localization and expression of putative circadian clock transcripts in the brain of the nudibranch Melibe leonina.Comp Biochem Physiol A Mol Integr Physiol. 2018 Sep;223:52-59. doi: 10.1016/j.cbpa.2018.05.002. Epub 2018 May 9. Comp Biochem Physiol A Mol Integr Physiol. 2018. PMID: 29753034 Free PMC article.
-
Clocks at a snail pace: biological rhythms in terrestrial gastropods.PeerJ. 2024 Oct 29;12:e18318. doi: 10.7717/peerj.18318. eCollection 2024. PeerJ. 2024. PMID: 39494278 Free PMC article. Review.
References
-
- Ajeska RA, Nybakken J. Contributions to the biology of Melibe leonina (Gould, 1852) (Mollusca; Opisthobranchia) Veliger. 1976;19:19–26.
-
- Arvanitaki A, Chalazonitas N. Excitatory and inhibitory processes initiated by light and infra-red radiations in single identifiable nerve cells (giant ganglion cells in Aplysia) In: Florey E, editor. Nervous Inhibition. Pergamon Press; New York: 1961. pp. 194–231.
-
- Aschoff J. Cold Spring Harbor Symposia on Quantitative Biology. Vol. 25, Biological Clocks. Long Island Biological Association; Cold Spring Harbor, NY: 1960. Exogenous and endogenous components in circadian rhythms; pp. 11–28. - PubMed
-
- Barsby T, Linington RG, Andersen RJ. De Novo terpenoid biosynthesis by the dendronotid nudibranch Melibe leonina. Chemoecology. 2002;12:199–202.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

