Chemotactic response with a constant delay-time mechanism in Ciona spermatozoa revealed by a high time resolution analysis of flagellar motility
- PMID: 25572419
- PMCID: PMC4365479
- DOI: 10.1242/bio.20137351
Chemotactic response with a constant delay-time mechanism in Ciona spermatozoa revealed by a high time resolution analysis of flagellar motility
Abstract
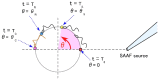
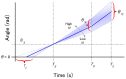
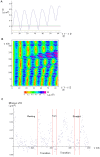
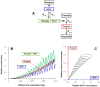
During their chemotactic swimming toward eggs, sperm cells detect their species-specific chemoattractant and sense concentration gradients by unknown mechanisms. After sensing the attractant, sperm cells commonly demonstrate a series of responses involving different swimming patterns by changing flagellar beats, gradually approaching a swimming path toward the eggs, which is the source of chemoattractants. Shiba et al. observed a rapid increase in intracellular Ca(2+) concentrations in Ciona spermatozoa after sensing chemoattractants; however, the biochemical processes occurring inside the sperm cells are unclear. In the present study, we focused on the timing and sensing mechanism of chemical signal detection in Ciona. One of the most crucial problems to be solved is defining the initial epoch of chemotactic responses. We adopted a high rate of video recording (600 Hz) for detailed analysis of sperm motion and a novel method for detecting subtle signs of beat forms and moving paths of sperm heads. From these analyses, we estimated a virtual sensing point of the attractant before initiation of motility responses and found that the time delay from sensing to motility responses was almost constant. To evaluate the efficiency of this constant delay model, we performed computer simulation of chemotactic behaviors of Ciona spermatozoa.
Keywords: Ciona intestinalis; Constant delay model; Flagellar motility; Sperm chemotaxis.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
Figures


Similar articles
-
Ca2+ bursts occur around a local minimal concentration of attractant and trigger sperm chemotactic response.Proc Natl Acad Sci U S A. 2008 Dec 9;105(49):19312-7. doi: 10.1073/pnas.0808580105. Epub 2008 Dec 1. Proc Natl Acad Sci U S A. 2008. PMID: 19047630 Free PMC article.
-
The Role of Soluble Adenylyl Cyclase in the Regulation of Flagellar Motility in Ascidian Sperm.Biomolecules. 2023 Oct 30;13(11):1594. doi: 10.3390/biom13111594. Biomolecules. 2023. PMID: 38002275 Free PMC article.
-
The Roles of Two CNG Channels in the Regulation of Ascidian Sperm Chemotaxis.Int J Mol Sci. 2022 Jan 31;23(3):1648. doi: 10.3390/ijms23031648. Int J Mol Sci. 2022. PMID: 35163568 Free PMC article.
-
Sperm chemotaxis and regulation of flagellar movement by Ca2+.Mol Hum Reprod. 2011 Aug;17(8):457-65. doi: 10.1093/molehr/gar041. Epub 2011 May 24. Mol Hum Reprod. 2011. PMID: 21610215 Review.
-
Tuning sperm chemotaxis.Biochem Soc Trans. 2010 Oct;38(5):1270-4. doi: 10.1042/BST0381270. Biochem Soc Trans. 2010. PMID: 20863297 Review.
Cited by
-
Ca2+ efflux via plasma membrane Ca2+-ATPase mediates chemotaxis in ascidian sperm.Sci Rep. 2018 Nov 9;8(1):16622. doi: 10.1038/s41598-018-35013-2. Sci Rep. 2018. PMID: 30413746 Free PMC article.
-
CatSper mediates not only chemotactic behavior but also the motility of ascidian sperm.Front Cell Dev Biol. 2023 Nov 2;11:1136537. doi: 10.3389/fcell.2023.1136537. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38020915 Free PMC article.
-
Sperm navigation along helical paths in 3D chemoattractant landscapes.Nat Commun. 2015 Aug 17;6:7985. doi: 10.1038/ncomms8985. Nat Commun. 2015. PMID: 26278469 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

