Positron emission tomography-adapted therapy for first-line treatment in individuals with Hodgkin lymphoma
- PMID: 25572491
- PMCID: PMC11064763
- DOI: 10.1002/14651858.CD010533.pub2
Positron emission tomography-adapted therapy for first-line treatment in individuals with Hodgkin lymphoma
Update in
-
Positron emission tomography-adapted therapy for first-line treatment in adults with Hodgkin lymphoma.Cochrane Database Syst Rev. 2025 Mar 26;3(3):CD010533. doi: 10.1002/14651858.CD010533.pub3. Cochrane Database Syst Rev. 2025. PMID: 40135712
Abstract
Background: Hodgkin lymphoma (HL) is a B-cell lymphoma accounting for 10% to 15% of all lymphoma in industrialised countries. It has a bimodal age distribution with one peak around the age of 30 years and another after the age of 60 years. Although HL accounts for fewer than 1% of all neoplasms worldwide, it is considered to be one of the most common malignancies in young adults and, with cure rates of 90%, one of the most curable cancers worldwide. Current treatment options for HL comprise more- or less-intensified regimens of chemotherapy plus radiotherapy, depending on disease stage. [18F]-fluorodeoxy-D-glucose (FDG)-positron emission tomography (PET, also called PET scanning) is an imaging tool that can be used to illustrate a tumour's metabolic activity, stage and progression. Therefore, it could be used as a standard interim procedure during HL treatment, to help distinguish between individuals who are good or poor early responders to therapy. Subsequent therapy could then be de-escalated in PET-negative individuals (good responders) or escalated in those who are PET-positive (poor responders). It is currently unknown whether such response-adapted therapeutic strategies are of benefit to individuals in terms of overall and progression-free survival, and the incidence of long-term adverse events (AEs).
Objectives: To assess the effects of interim [18F]-FDG-PET imaging treatment modification in individuals with HL.
Search methods: We searched the Cochrane Central Register of Controlled Trials (CENTRAL; latest issue) and MEDLINE (from 1990 to September 2014) as well as conference proceedings (American Society of Hematology; American Society of Clinical Oncology; European Hematology Association; and International Symposium on Hodgkin Lymphoma) for studies. Two review authors independently screened search results.
Selection criteria: We included randomised controlled trials (RCTs) comparing FDG-PET-adapted therapy with non-adapted treatment in individuals with previously untreated HL of all stages and ages.
Data collection and analysis: Two review authors independently extracted data and assessed the quality of trials. As none of the included studies provided HRs for OS, we described risk ratios (RRs) for this outcome and did not pool the data. As an effect measure we used hazard ratios (HRs) for progression-free survival (PFS). We described RRs for the dichotomous data on AEs. We also calculated 95% confidence intervals (CIs).
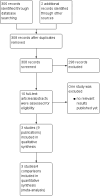
Main results: Our search strategies led to 308 potentially relevant references. From these, we included three studies involving 1999 participants. We judged the overall potential risk of bias as moderate. The studies were reported as RCTs; blinding was not reported, but given the study design it is likely that there was no blinding. One study was published in abstract form only; hence, detailed assessment of the risk of bias was not possible.Two trials compared standard treatment (chemotherapy plus radiotherapy) with PET-adapted therapy (chemotherapy only) in individuals with early-stage HL and negative PET scans. The study design of the third trial was more complex. Participants with early-stage HL were divided into those with a favourable or unfavourable prognosis. They were then randomised to receive PET-adapted or standard treatment. Following a PET scan, participants were further divided into PET-positive and PET-negative groups. To date, data have been published for the PET-negative arms only, making it possible to perform a meta-analysis including all three trials.Of the 1999 participants included in the three trials only 1480 were analysed. The 519 excluded participants were either PET-positive, or were excluded because they did not match the inclusion criteria.One study reported no deaths. The other two studies reported two deaths in participants receiving PET-adapted therapy and two in participants receiving standard therapy (very-low-quality evidence). Progression-free survival was shorter in participants with PET-adapted therapy (without radiotherapy) than in those receiving standard treatment with radiotherapy (HR 2.38; 95% CI 1.62 to 3.50; P value < 0.0001). This difference was also apparent in comparisons of participants receiving no additional radiotherapy (PET-adapted therapy) versus radiotherapy (standard therapy) (HR 1.86; 95% CI 1.07 to 3.23; P value = 0.03) and in those receiving chemotherapy but no radiotherapy (PET-adapted therapy) versus standard radiotherapy (HR 3.00; 95% CI 1.75 to 5.14; P value < 0.0001) (moderate-quality evidence). Short-term AEs only were assessed in one trial, which showed no evidence of a difference between the treatment arms (RR 0.91; 95% CI 0.54 to 1.53; P value = 0.72) (very-low-quality evidence). No data on long-term AEs were reported in any of the trials.
Authors' conclusions: To date, no robust data on OS, response rate, TRM, QoL, or short- and long-term AEs are available. However, this systematic review found moderate-quality evidence that PFS was shorter in individuals with early-stage HL and a negative PET scan receiving chemotherapy only (PET-adapted therapy) than in those receiving additional radiotherapy (standard therapy). More RCTs with longer follow ups may lead to more precise results for AEs, TRM and QoL, and could evaluate whether this PFS advantage will translate into an overall survival benefit.It is still uncertain whether PET-positive individuals benefit from PET-based treatment adaptation and the effect of such an approach in those with advanced HL.
Conflict of interest statement
None
Figures


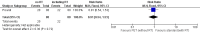





References
References to studies included in this review
H10F {published data only}
-
- Andre MPE. An update on the EORTC/LYSA/FIL H10 trial. 9th International Symposium on Hodgkin Lymphoma Cologne, Germany 2013.
-
- Andre MPE, Reman O, Federico M, Girinski T, Brice P, Brusamolino E, et al. Interim analysis of the randomized EORTC/LYSA/FIl Intergroup H10 trial on early PET‐scan driven treatment adaptation in stage I/II Hodgkin lymphoma [abstract]. Blood. 2012; Vol. 120:549.
-
- Raemaekers JM, Andre MP, Federico M, Girinsky T, Oumedaly R, Brusamolino E, et al. Omitting radiotherapy in early positron emission tomography‐negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. Journal of Clinical Oncology 2014;32(12):1188‐94. [PUBMED: 24637998] - PubMed
H10U {published data only}
-
- Andre MPE. An update on the EORTC/LYSA/FIL H10 trial. 9th International Symposium on Hodgkin Lymphoma Cologne, Germany 2013.
-
- Andre MPE, Reman O, Federico M, Girinski T, Brice P, Brusamolino E, et al. Interim analysis of the randomized EORTC/LYSA/FIl Intergroup H10 trial on early PET‐scan driven treatment adaptation in stage I/II Hodgkin lymphoma [abstract]. Blood. 2012; Vol. 120:549.
-
- Raemaekers JM, Andre MP, Federico M, Girinsky T, Oumedaly R, Brusamolino E, et al. Omitting radiotherapy in early positron emission tomography‐negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. Journal of Clinical Oncology 2014;32(12):1188‐94. [PUBMED: 24637998] - PubMed
Picardi {published data only}
-
- Picardi M, Renzo A, Pane F, Nicolai E, Pacelli R, Salvatore M, et al. Randomized comparison of consolidation radiation versus observation in bulky Hodgkin's lymphoma with post‐chemotherapy negative positron emission tomography scans. Leukemia & Lymphoma 2007;48:1721‐7. - PubMed
UK NCRI RAPID {published data only}
-
- Radford J. Update on the NCRI RAPID trial. 9th International Symposium on Hodgkin Lymphoma, Cologne, Germany 2013.
-
- Radford J, Barrington S, Counsell N, Pettengell R, Johnson P, Wimperis J, et al. Involved field radiotherapy versus no further treatment in patients with clinical stages IA and IIA Hodgkin lymphoma and a 'negative' PET scan after 3 cycles ABVD. Results of the UK NCRI RAPID trial [abstract]. Blood. 2012; Vol. 120:547.
-
- Radford J, O'Doherty M, Barrington S, Qian W, Patrick P, Coltart S, et al. Results of the 2nd planned interim analysis of the rapid trial (involved field radiotherapy versus no further treatment) in patients with clinical stages 1a and 2a Hodgkin lymphoma and a 'negative' FDG‐PET scan after 3 cycles ABVD [abstract 369]. Blood. 2009; Vol. 112:143‐4.
-
- Radford J, O'Doherty M, Barrington S, Qian W, Popova B, Pettengell R, et al. Results of the 3rd planned interim analysis of the UK NCRI rapid trial (involved field radiotherapy versus no further treatment) in patients with clinical stages IA/IIA Hodgkin lymphoma and a 'negative' 18 FDG‐PET scan after 3 cycles ABVD [Abstract P059]. Haematologica 2010;95:16‐7.
-
- Radford JA, Barrington SF, O'Doherty MJ, Qian W, Mouncey P, Pettengell R, et al. Interim results of a UK NCRI randomized trial comparing involved field radiotherapy with no further treatment after 3 cycles ABVD and a negative pet scan in clinical stages IA/IIA Hodgkin lymphoma [abstract C023]. Haematologica. 2007; Vol. 92:32.
References to studies excluded from this review
HD 0607 {published data only}
-
- Gallamini A, Rossi A, Patti C, Picardi M, Raimondo F, Cantonetti M, et al. Early treatment intensification in advanced‐stage high‐risk Hodgkin lymphoma (HL) patients, with a positive FDG‐PET scan after two ABVD courses ‐ first interim analysis of the GITIL/FIL HD0607 clinical trial [Abstract 550]. Blood (ASH Annual Meeting Abstracts) 2012;120:550.
References to ongoing studies
Casanovas PHRC N 2010 {published data only}
-
- Randomised phase III study of a treatment driven by early PET response compared with a treatment not monitored by early PET in individuals with Ann Arbor stage III‐IV or high‐risk IIB HL. Ongoing study May 2011.
CRUK‐07/146 {published data only}
-
- A randomised phase III trial to assess response‐adapted therapy using FDG‐PET imaging in individuals with newly diagnosed, advanced HL. Ongoing study August 2008.
EU‐20931 {published data only}
-
- A randomised phase III trial to determine the role of FDG‐PET imaging in clinical stages IA/IIA Hodgkin's disease. Ongoing study July 2003.
HD0607 {published data only}
-
- PET‐adapted chemotherapy in advanced HL. Ongoing study June 2008.
HD 18 {published data only}
-
- HD18 for advanced stages in HL. Ongoing study May 2008.
Additional references
Bauer 2011
-
- Bauer K, Skoetz N, Monsef I, Engert A, Brillant C. Comparison of chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for patients with early unfavourable or advanced stage Hodgkin lymphoma. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD007941.pub2] - DOI - PubMed
Boellaard 2010
Borchmann 2011
-
- Borchmann P, Haverkamp H, Diehl V, Cerny T, Markova J, Ho AD, et al. Eight cycles of escalated‐dose BEACOPP compared with four cycles of escalated‐dose BEACOPP followed by four cycles of baseline‐dose BEACOPP with or without radiotherapy in patients with advanced‐stage Hodgkin's lymphoma: final analysis of the HD12 trial of the German Hodgkin Study Group. Journal of Clinical Oncology 2011;29(32):4234‐42. [PUBMED: 21990399] - PubMed
Canellos 1992
-
- Canellos GP, Anderson JR, Propert KJ, Nissen N, Cooper MR, Henderson ES, et al. Chemotherapy of advanced Hodgkin's disease with MOPP, ABVD, or MOPP alternating with ABVD. The New England Journal of Medicine 1992;327(21):1478‐84. [PUBMED: 1383821] - PubMed
Connors 2009
-
- Connors JM. Clinical manifestations and natural history of Hodgkin's lymphoma . Cancer Journal 2009;15(2):124‐8. - PubMed
Deeks 2011
-
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Diaz 2011
Diehl 2001
-
- Diehl V, Mauch P, Harris NL. Hodgkin's disease. In: Vita V editor(s). Cancer Principles and Practice of Oncology. Philadelphia: Lippincott, Williams and Wilkins, 2001:2339‐87.
Engert 2003
-
- Engert A, Schiller P, Josting A, Herrmann R, Koch P, Sieber M, et al. Involved‐field radiotherapy is equally effective and less toxic compared with extended‐field radiotherapy after four cycles of chemotherapy in patients with early‐stage unfavorable Hodgkin's lymphoma: results of the HD8 trial of the German Hodgkin's Lymphoma Study Group. Journal of Clinical Oncology 2003;21(19):3601‐8. - PubMed
Engert 2007
-
- Engert A, Franklin J, Eich HT, Brillant C, Sehlen S, Cartoni C, et al. Two cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine plus extended‐field radiotherapy is superior to radiotherapy alone in early favorable Hodgkin's lymphoma: final results of the GHSG HD7 trial. Journal of Clinical Oncology 2007;25(23):3495‐502. - PubMed
Engert 2010
-
- Engert A, Plutschow A, Eich HT, Lohri A, Dorken B, Borchmann P, et al. Reduced treatment intensity in patients with early‐stage Hodgkin's lymphoma. The New England Journal of Medicine 2010;363(7):640‐52. [PUBMED: 20818855] - PubMed
Engert 2012
-
- Engert A, Haverkamp H, Kobe C, Markova J, Renner C, Ho A, et al. Reduced‐intensity chemotherapy and PET‐guided radiotherapy in patients with advanced stage Hodgkin's lymphoma (HD15 trial): a randomised, open‐label, phase 3 non‐inferiority trial. Lancet 2012;379(9828):1791‐9. [PUBMED: 22480758] - PubMed
Fraga 2007
-
- Fraga M, Forteza J. Diagnosis of Hodgkin's disease: an update on histopathological and immunophenotypical features. Histology and Histopathology 2007;22(8):923‐35. [PUBMED: 17503349] - PubMed
Gallamini 2007
-
- Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, et al. Early interim 2‐[18F]fluoro‐2‐deoxy‐D‐glucose positron emission tomography is prognostically superior to international prognostic score in advanced‐stage Hodgkin's lymphoma: a report from a joint Italian‐Danish study. Journal of Clinical Oncology 2007;25(24):3746‐52. [PUBMED: 17646666] - PubMed
Harris 1999
-
- Harris NL. Hodgkin's lymphomas: classification, diagnosis, and grading. Seminars in Hematology 1999;36(3):220‐32. [PUBMED: 10462322] - PubMed
Higgins 2011a
-
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
-
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Klimm 2005
-
- Klimm B, Engert A, Diehl V. First‐line treatment of Hodgkin's lymphoma. Current Hematology Reports 2005;4(1):15‐22. - PubMed
Kobe 2008
-
- Kobe C, Dietlein M, Franklin J, Markova J, Lohri A, Amthauer H, et al. Positron emission tomography has a high negative predictive value for progression or early relapse for patients with residual disease after first‐line chemotherapy in advanced‐stage Hodgkin lymphoma. Blood 2008;112(10):3989‐94. [PUBMED: 18757777] - PMC - PubMed
Kobe 2010a
-
- Kobe C, Dietlein M, Kriz J, Furth C, Fuchs M, Borchmann P, et al. The role of PET in Hodgkin's lymphoma and its impact on radiation oncology. Expert Review of Anticancer Therapy 2010;10(9):1419‐28. [PUBMED: 20836677] - PubMed
Kobe 2010b
-
- Kobe C, Dietlein M, Fuchs M. Interpretation and validation of interim positron emission tomography in Hodgkin lymphoma. Leukemia & Lymphoma 2010; Vol. 51, issue 3:552‐3. [PUBMED: 20141443] - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lister 1989
-
- Lister TA, Crowther D, Sutcliffe SB, Glatstein E, Canellos GP, Young RC, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. Journal of Clinical Oncology 1989;7(11):1630‐6. - PubMed
Markova 2009
-
- Markova J, Kobe C, Skopalova M, Klaskova K, Dedeckova K, Plutschow A, et al. FDG‐PET for assessment of early treatment response after four cycles of chemotherapy in patients with advanced‐stage Hodgkin's lymphoma has a high negative predictive value. Annals of Oncology 2009;20(7):1270‐4. [PUBMED: 19228806] - PubMed
Markova 2012
-
- Markova J, Kahraman D, Kobe C, Skopalova M, Mocikova H, Klaskova K, et al. Role of [18F]‐fluoro‐2‐deoxy‐D‐glucose positron emission tomography in early and late therapy assessment of patients with advanced Hodgkin lymphoma treated with bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine and prednisone. Leukemia & Lymphoma 2012;53(1):64‐70. [PUBMED: 21740300] - PubMed
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. [PUBMED: 19631508] - PubMed
Oken 1982
-
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology 1982;5(6):649‐55. [PUBMED: 7165009] - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [PUBMED: 9921604] - PubMed
Pileri 2002
Rancea 2013
Re 2005
-
- Re D, Thomas RK, Behringer K, Diehl V. From Hodgkin disease to Hodgkin lymphoma: biologic insights and therapeutic potential. Blood 2005;105(12):4553‐60. [PUBMED: 15728122] - PubMed
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings tables'. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Shenoy 2011
Specht 2007
-
- Specht L. FDG‐PET scan and treatment planning for early stage Hodgkin lymphoma. Radiotherapy and Oncology 2007; Vol. 85, issue 2:176‐7. [PUBMED: 17920143] - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Intervention. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Thomas 2002
-
- Thomas RK, Re D, Zander T, Wolf J, Diehl V. Epidemiology and etiology of Hodgkin's lymphoma. Annals of Oncology 2002;13 Suppl 4:147‐52. [PUBMED: 12401681] - PubMed
Tierney 2007
von Tresckow 2012
-
- Tresckow B, Plutschow A, Fuchs M, Klimm B, Markova J, Lohri A, et al. Dose‐intensification in early unfavorable Hodgkin's lymphoma: final analysis of the German Hodgkin study group HD14 trial. Journal of Clinical Oncology 2012;30(9):907‐13. [PUBMED: 22271480] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

