S-1 plus cisplatin with concurrent radiotherapy versus cisplatin alone with concurrent radiotherapy for stage III non-small cell lung cancer: a pilot randomized controlled trial
- PMID: 25572571
- PMCID: PMC4311504
- DOI: 10.1186/s13014-014-0306-3
S-1 plus cisplatin with concurrent radiotherapy versus cisplatin alone with concurrent radiotherapy for stage III non-small cell lung cancer: a pilot randomized controlled trial
Abstract
Background: We investigated the efficacy and safety of S-1 and cisplatin with concurrent thoracic radiation (SCCR) over cisplatin alone plus concurrent thoracic radiation (CCR) for unresectable stage III non-small-cell lung cancer (NSCLC).
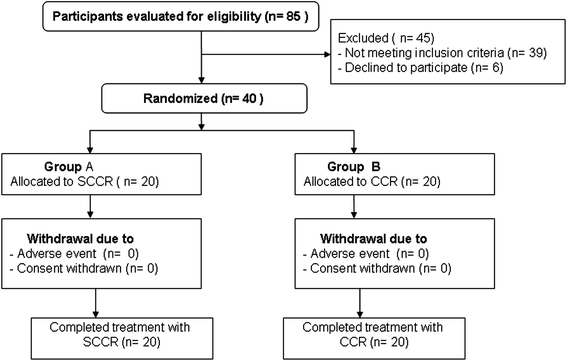
Methods: Between January 2009 and November 2011, 40 eligible patients with NSCLC were included and divided randomly into two groups. Twenty patients received SCCR with S-1 (orally at 40 mg/m(2) per dose, b.i.d.) on days 1 through 14, cisplatin (60 mg/m(2) on day 1) every 4 weeks for two cycles, and radiotherapy (60 Gy/30 fractions over 6 weeks) beginning on day 1. Twenty subjects received CCR (cisplatin and radiotherapy, the same as for SCCR).
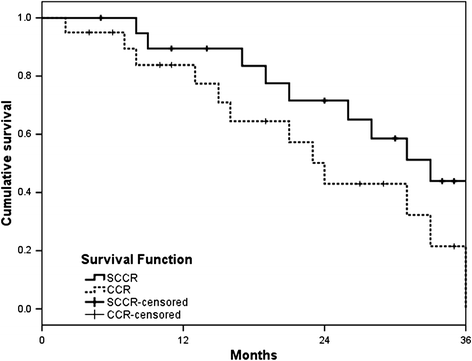
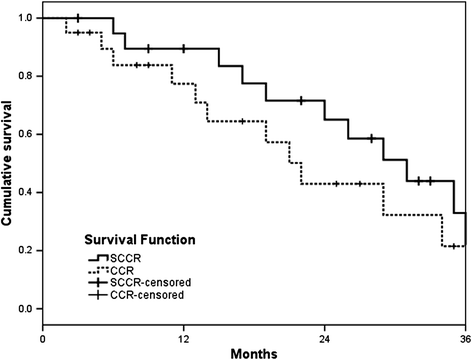
Results: The 3-year overall response rate was 59.3% and 52.4% for the SCCR and CCR groups, respectively, and the difference was statistically significant, while the median overall survival was 33 months (range, 4-41 months) and 24 months (range, 2-37 months), respectively (P = 0.048). The median progression-free survival was 31 months for SCCR (range, 5-39 months), whereas it was 20 months (range, 2-37 months) for CCR (P = 0.037). The toxicity profile was similar in both groups.
Conclusion: In summary, we demonstrated that S-1 and cisplatin with concurrent thoracic radiation was more effective than cisplatin plus radiotherapy in NSCLC patients with acceptable toxicity.
Trial registration: Chinese Clinical Trials Register: ChiCTR-TRC-13003997 .
Figures
Similar articles
-
Dose-escalation study of three-dimensional conformal thoracic radiotherapy with concurrent S-1 and cisplatin for inoperable stage III non-small-cell lung cancer.Clin Lung Cancer. 2013 Jul;14(4):440-5. doi: 10.1016/j.cllc.2013.01.003. Epub 2013 Mar 27. Clin Lung Cancer. 2013. PMID: 23540866 Clinical Trial.
-
Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study.Lancet Oncol. 2015 Feb;16(2):187-99. doi: 10.1016/S1470-2045(14)71207-0. Epub 2015 Jan 16. Lancet Oncol. 2015. PMID: 25601342 Free PMC article. Clinical Trial.
-
Phase 2 study of pemetrexed plus carboplatin, or pemetrexed plus cisplatin with concurrent radiation therapy followed by pemetrexed consolidation in patients with favorable-prognosis inoperable stage IIIA/B non-small-cell lung cancer.J Thorac Oncol. 2013 Oct;8(10):1308-16. doi: 10.1097/JTO.0b013e3182a02546. J Thorac Oncol. 2013. PMID: 23981966 Free PMC article. Clinical Trial.
-
A randomized phase III trial of oral S-1 plus cisplatin versus docetaxel plus cisplatin in Japanese patients with advanced non-small-cell lung cancer: TCOG0701 CATS trial.Ann Oncol. 2015 Jul;26(7):1401-8. doi: 10.1093/annonc/mdv190. Epub 2015 Apr 23. Ann Oncol. 2015. PMID: 25908605 Free PMC article. Clinical Trial.
-
Randomized phase II trial of cisplatin, etoposide, and radiation followed by gemcitabine alone or by combined gemcitabine and docetaxel in stage III A/B unresectable non-small cell lung cancer.J Thorac Oncol. 2010 May;5(5):673-9. doi: 10.1097/JTO.0b013e3181d60e8f. J Thorac Oncol. 2010. PMID: 20354453 Clinical Trial.
Cited by
-
A randomised phase II trial of S-1 plus cisplatin versus vinorelbine plus cisplatin with concurrent thoracic radiotherapy for unresectable, locally advanced non-small cell lung cancer: WJOG5008L.Br J Cancer. 2018 Sep;119(6):675-682. doi: 10.1038/s41416-018-0243-2. Epub 2018 Sep 12. Br J Cancer. 2018. PMID: 30206369 Free PMC article. Clinical Trial.
-
A network meta-analysis for efficacies and toxicities of different concurrent chemoradiotherapy regimens in the treatment of locally advanced non-small cell lung cancer.BMC Cancer. 2022 Jun 20;22(1):674. doi: 10.1186/s12885-022-09717-8. BMC Cancer. 2022. PMID: 35725420 Free PMC article.
-
S-1-based concurrent chemoradiotherapy in the treatment of locally advanced non-small cell lung cancer: A systematic review and meta-analysis protocol.Medicine (Baltimore). 2018 Apr;97(15):e0397. doi: 10.1097/MD.0000000000010397. Medicine (Baltimore). 2018. PMID: 29642202 Free PMC article.
-
Concurrent chemoradiotherapy with cisplatin + S-1 versus cisplatin + other third-generation agents for locally advanced non-small-cell lung cancer: a meta-analysis of individual participant data.BMC Pulm Med. 2022 Jan 9;22(1):31. doi: 10.1186/s12890-022-01828-z. BMC Pulm Med. 2022. PMID: 35000608 Free PMC article.
-
Comparison of post-chemoradiotherapy pneumonitis between Asian and non-Asian patients with locally advanced non-small cell lung cancer: a systematic review and meta-analysis.EClinicalMedicine. 2023 Sep 25;64:102246. doi: 10.1016/j.eclinm.2023.102246. eCollection 2023 Oct. EClinicalMedicine. 2023. PMID: 37781162 Free PMC article.
References
-
- Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small cell lung cancer. J Clin Oncol. 1999;17:2692–9. - PubMed
-
- Curran W, Scott C, Langer C. Phase III comparison of sequential vs concurrent chemoradiation for patients with unresected stage III non-small cell lung cancer: report of Radiation Therapy Oncology Group (RTOG) 9410. Lung Cancer. 2000;29(Suppl 1):93. doi: 10.1016/S0169-5002(00)80304-9. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical