Glial cell line-derived neurotrophic factor-mediated enhancement of noradrenergic descending inhibition in the locus coeruleus exerts prolonged analgesia in neuropathic pain
- PMID: 25572945
- PMCID: PMC4409900
- DOI: 10.1111/bph.13073
Glial cell line-derived neurotrophic factor-mediated enhancement of noradrenergic descending inhibition in the locus coeruleus exerts prolonged analgesia in neuropathic pain
Abstract
Background and purpose: The locus coeruleus (LC) is the principal nucleus containing the noradrenergic neurons and is a major endogenous source of pain modulation in the brain. Glial cell line-derived neurotrophic factor (GDNF), a well-established neurotrophic factor for noradrenergic neurons, is a major pain modulator in the spinal cord and primary sensory neurons. However, it is unknown whether GDNF is involved in pain modulation in the LC.
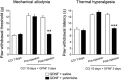
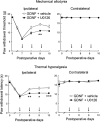
Experimental approach: Rats with chronic constriction injury (CCI) of the left sciatic nerve were used as a model of neuropathic pain. GDNF was injected into the left LC of rats with CCI for 3 consecutive days and changes in mechanical allodynia and thermal hyperalgesia were assessed. The α2 -adrenoceptor antagonist yohimbine was injected intrathecally to assess the involvement of descending inhibition in GDNF-mediated analgesia. The MEK inhibitor U0126 was used to investigate whether the ERK signalling pathway plays a role in the analgesic effects of GDNF.
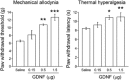
Key results: Both mechanical allodynia and thermal hyperalgesia were attenuated 24 h after the first GDNF injection. GDNF increased the noradrenaline content in the dorsal spinal cord. The analgesic effects continued for at least 3 days after the last injection. Yohimbine abolished these effects of GDNF. The analgesic effects of GDNF were partly, but significantly, inhibited by prior injection of U0126 into the LC.
Conclusions and implications: GDNF injection into the LC exerts prolonged analgesic effects on neuropathic pain in rats by enhancing descending noradrenergic inhibition.
© 2015 The British Pharmacological Society.
Figures


Similar articles
-
Activation of NK₁ receptors in the locus coeruleus induces analgesia through noradrenergic-mediated descending inhibition in a rat model of neuropathic pain.Br J Pharmacol. 2012 Jun;166(3):1047-57. doi: 10.1111/j.1476-5381.2011.01820.x. Br J Pharmacol. 2012. PMID: 22188400 Free PMC article.
-
Repeated Administration of Amitriptyline in Neuropathic Pain: Modulation of the Noradrenergic Descending Inhibitory System.Anesth Analg. 2017 Oct;125(4):1281-1288. doi: 10.1213/ANE.0000000000002352. Anesth Analg. 2017. PMID: 28787345
-
GABA-A receptor activity in the noradrenergic locus coeruleus drives trigeminal neuropathic pain in the rat; contribution of NAα1 receptors in the medial prefrontal cortex.Neuroscience. 2016 Oct 15;334:148-159. doi: 10.1016/j.neuroscience.2016.08.005. Epub 2016 Aug 9. Neuroscience. 2016. PMID: 27520081 Free PMC article.
-
Analgesic Mechanisms of Antidepressants for Neuropathic Pain.Int J Mol Sci. 2017 Nov 21;18(11):2483. doi: 10.3390/ijms18112483. Int J Mol Sci. 2017. PMID: 29160850 Free PMC article. Review.
-
[Mechanisms of antidepressants and serotonin (5-HT)-induced glial cell line-derived neurotrophic factor (GDNF) releases in rat C6 gliobrastoma cells].Nihon Shinkei Seishin Yakurigaku Zasshi. 2005 Feb;25(1):25-31. Nihon Shinkei Seishin Yakurigaku Zasshi. 2005. PMID: 15796067 Review. Japanese.
Cited by
-
The Non-Survival Effects of Glial Cell Line-Derived Neurotrophic Factor on Neural Cells.Front Mol Neurosci. 2017 Aug 22;10:258. doi: 10.3389/fnmol.2017.00258. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28878618 Free PMC article. Review.
-
A neuroanatomical framework for the central modulation of respiratory sensory processing and cough by the periaqueductal grey.J Thorac Dis. 2017 Oct;9(10):4098-4107. doi: 10.21037/jtd.2017.08.119. J Thorac Dis. 2017. PMID: 29268420 Free PMC article. Review.
-
Locus coeruleus-noradrenergic modulation of trigeminal pain: Implications for trigeminal neuralgia and psychiatric comorbidities.Neurobiol Pain. 2023 Mar 20;13:100124. doi: 10.1016/j.ynpai.2023.100124. eCollection 2023 Jan-Jul. Neurobiol Pain. 2023. PMID: 36974102 Free PMC article.
-
Fentanyl vs fentanyl-dexmedetomidine in lumbar foraminotomy surgery.Ther Clin Risk Manag. 2019 Jul 15;15:885-890. doi: 10.2147/TCRM.S195108. eCollection 2019. Ther Clin Risk Manag. 2019. PMID: 31406463 Free PMC article.
-
Interplay of BDNF and GDNF in the Mature Spinal Somatosensory System and Its Potential Therapeutic Relevance.Curr Neuropharmacol. 2021;19(8):1225-1245. doi: 10.2174/1570159X18666201116143422. Curr Neuropharmacol. 2021. PMID: 33200712 Free PMC article. Review.
References
-
- Ackerman LL, Follett KA, Rosenquist RW. Long-term outcomes during treatment of chronic pain with intrathecal clonidine or clonidine/opioid combinations. J Pain Symptom Manage. 2003;26:668–677. - PubMed
-
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. - PubMed
-
- Alba-Delgado C, Mico JA, Sánchez-Blázquez P, Berrocoso E. Analgesic antidepressants promote the responsiveness of locus coeruleus neurons to noxious stimulation: implications for neuropathic pain. Pain. 2012;153:1438–1449. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

