Combination treatment with triptolide and hydroxycamptothecin synergistically enhances apoptosis in A549 lung adenocarcinoma cells through PP2A-regulated ERK, p38 MAPKs and Akt signaling pathways
- PMID: 25573072
- PMCID: PMC4324588
- DOI: 10.3892/ijo.2015.2814
Combination treatment with triptolide and hydroxycamptothecin synergistically enhances apoptosis in A549 lung adenocarcinoma cells through PP2A-regulated ERK, p38 MAPKs and Akt signaling pathways
Abstract
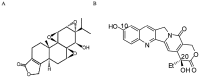
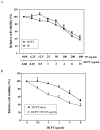
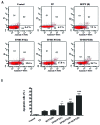
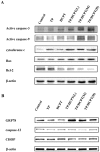
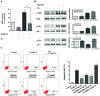
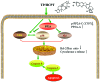
Lung cancer is the leading cause of cancer death worldwide. Recently, two plant-derived drugs triptolide (TP) and hydroxycamptothecin (HCPT) both have shown broad-spectrum anticancer activities. Our previous study documented that combination treatment with these two drugs acted more effectively than mono-therapy, however, the molecular basis underlying the synergistic cytotoxicity remains poorly understood. In this study, we aimed to clarify the molecular mechanism of TP/HCPT anticancer effect in A549 lung adenocarcinoma cells, by investigating the involvement of phosphatase 2A (PP2A) and PP2A-regulated mitogen-activated protein kinases (MAPKs) and Akt signaling pathways. The results showed that TP and HCPT synergistically exerted cytotoxicity in the growth of A549 cells. Combinatorial TP/HCPT treatment significantly enhanced the activation of caspase-3 and -9, Bax/Bcl-2 ratio, release of cytochrome c from mitochondrial and subsequent apoptosis. While the Akt survival pathway was inhibited, ERK and p38 MAPKs were dramatically activated. Furthermore, the activity of PP2A was significantly augmented. Regulation of p38, ERK and Akt by PP2A was demonstrated, by using a specific PP2A inhibitor okadaic acid (OA). Finally, pharmacological inhibitors OA, SB203580, SP600125 and PD98059 confirm the role of PP2A and its substrates ERK, p38 MAPK and Akt in mediating TP/HCPT-induced apoptosis. Taken together, this study provides the first evidence for a synergistic TP/HCPT anticancer activity in A549 cells and also supports a critical role of PP2A and PP2A-regulated signaling pathways, providing new insight into the mode of action of TP/HCPT in cancer therapy.
Figures

Similar articles
-
Chemosensitizing effect of Paris Saponin I on Camptothecin and 10-hydroxycamptothecin in lung cancer cells via p38 MAPK, ERK, and Akt signaling pathways.Eur J Med Chem. 2017 Jan 5;125:760-769. doi: 10.1016/j.ejmech.2016.09.066. Epub 2016 Sep 23. Eur J Med Chem. 2017. PMID: 27721159
-
ITRAQ-Based Proteomics Analysis of Triptolide On Human A549 Lung Adenocarcinoma Cells.Cell Physiol Biochem. 2018;45(3):917-934. doi: 10.1159/000487286. Epub 2018 Feb 2. Cell Physiol Biochem. 2018. PMID: 29428961
-
Synergistic antitumour effects of triptolide plus 10-hydroxycamptothecin onbladder cancer.Biomed Pharmacother. 2019 Jul;115:108899. doi: 10.1016/j.biopha.2019.108899. Epub 2019 May 4. Biomed Pharmacother. 2019. PMID: 31063955
-
Protein Phosphatase 2A as a Therapeutic Target in Acute Myeloid Leukemia.Front Oncol. 2016 Apr 6;6:78. doi: 10.3389/fonc.2016.00078. eCollection 2016. Front Oncol. 2016. PMID: 27092295 Free PMC article. Review.
-
Therapeutic Potential of Natural Compounds in Lung Cancer.Curr Med Chem. 2021;28(39):7988-8002. doi: 10.2174/0929867328666210322103906. Curr Med Chem. 2021. PMID: 33749551 Review.
Cited by
-
Development of 10-Hydroxycamptothecin-crizotinib conjugate based on the synergistic effect on lung cancer cells.J Enzyme Inhib Med Chem. 2023 Dec;38(1):1-11. doi: 10.1080/14756366.2022.2132487. J Enzyme Inhib Med Chem. 2023. PMID: 36305251 Free PMC article.
-
Triptolide inhibits epithelial‑mesenchymal transition and induces apoptosis in gefitinib‑resistant lung cancer cells.Oncol Rep. 2020 May;43(5):1569-1579. doi: 10.3892/or.2020.7542. Epub 2020 Mar 10. Oncol Rep. 2020. PMID: 32323848 Free PMC article.
-
K9(C4H4FN2O2)2Nd(PW11O39)2·25H2O induces apoptosis in human lung cancer A549 cells.Oncol Lett. 2017 Mar;13(3):1348-1352. doi: 10.3892/ol.2016.5543. Epub 2016 Dec 28. Oncol Lett. 2017. PMID: 28454260 Free PMC article.
-
Neddylation inhibitor MLN4924 sensitizes head and neck squamous carcinoma cells to (S)-10-hydroxycamptothecin.Eur J Med Res. 2023 Sep 9;28(1):326. doi: 10.1186/s40001-023-01289-y. Eur J Med Res. 2023. PMID: 37689760 Free PMC article.
-
A Mechanistic Overview of Triptolide and Celastrol, Natural Products from Tripterygium wilfordii Hook F.Front Pharmacol. 2018 Feb 14;9:104. doi: 10.3389/fphar.2018.00104. eCollection 2018. Front Pharmacol. 2018. PMID: 29491837 Free PMC article. Review.
References
-
- Rajeswaran A, Trojan A, Burnand B, Giannelli M. Efficacy and side effects of cisplatin- and carboplatin-based doublet chemotherapeutic regimens versus non-platinum-based doublet chemotherapeutic regimens as first line treatment of metastatic non-small cell lung carcinoma: a systematic review of randomized controlled trials. Lung Cancer. 2008;59:1–11. doi: 10.1016/j.lungcan.2007.07.012. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

