GPCR dimerization in brainstem nuclei contributes to the development of hypertension
- PMID: 25573074
- PMCID: PMC4409903
- DOI: 10.1111/bph.13074
GPCR dimerization in brainstem nuclei contributes to the development of hypertension
Abstract
Background and purpose: μ-Opioid receptors, pro-opiomelanocortin and pro-enkephalin are highly expressed in the nucleus tractus solitarii (NTS) and μ receptor agonists given to the NTS dose-dependently increased BP. However, the molecular mechanisms of this process remain unclear. In vitro, μ receptors heterodimerize with α2A -adrenoceptors. We hypothesized that α2A -adrenoceptor agonists would lose their depressor effects when their receptors heterodimerize in the NTS with μ receptors.
Experimental approach: We microinjected μ-opioid agonists and antagonists into the NTS of rats and measured changes in BP. Formation of μ receptor/α2A -adrenoceptor heterodimers was assessed with immunofluorescence and co-immunoprecipitation methods, along with proximity ligation assays.
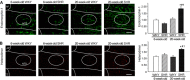
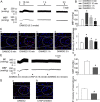
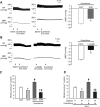
Key results: Immunofluorescence staining revealed colocalization of α2A -adrenoceptors and μ receptors in NTS neurons. Co-immunoprecipitation revealed interactions between α2A -adrenoceptors and μ receptors. In situ proximity ligation assays confirmed the presence of μ receptor/α2A -adrenoceptor heterodimers in the NTS. Higher levels of endogenous endomorphin-1 and μ receptor/α2A -adrenoceptor heterodimers were found in the NTS of hypertensive rats, than in normotensive rats. Microinjection of the μ receptor agonist [D-Ala(2) , MePhe(4) , Gly(5) -ol]-enkephalin (DAMGO), but not that of the α2A -adrenoceptor agonist guanfacine, into the NTS of normotensive rats increased μ receptor/α2A -adrenoceptor heterodimer formation and BP elevation. The NO-dependent BP-lowering effect of α2A -adrenoceptor agonists was blunted following increased formation of μ receptor/α2A -adrenoceptor heterodimers in the NTS of hypertensive rats and DAMGO-treated normotensive rats.
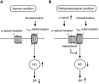
Conclusions and implications: Increases in endogenous μ receptor agonists in the NTS induced μ receptor/α2A -adrenoceptor heterodimer formation and reduced the NO-dependent depressor effect of α2A -adrenoceptor agonists. This process could contribute to the pathogenesis of hypertension.
© 2015 The British Pharmacological Society.
Figures


References
-
- AbdAlla S, Lother H, el Massiery A, Quitterer U. Increased AT(1) receptor heterodimers in preeclampsia mediate enhanced angiotensin II responsiveness. Nat Med. 2001;7:1003–1009. - PubMed
-
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, McGrath JC, et al. The Concise Guide to PHARMACOLOGY 2013/14: overview. Br J Pharmacol. 2013;170:1449–1458. - PubMed
-
- Altman JD, Trendelenburg AU, MacMillan L, Bernstein D, Limbird L, Starke K, et al. Abnormal regulation of the sympathetic nervous system in alpha2A-adrenergic receptor knockout mice. Mol Pharmacol. 1999;56:154–161. - PubMed
-
- Bhuiyan ME, Waki H, Gouraud SS, Takagishi M, Cui H, Yamazaki T, et al. Complex cardiovascular actions of alpha-adrenergic receptors expressed in the nucleus tractus solitarii of rats. Exp Physiol. 2009;94:773–784. - PubMed
-
- Brede M, Wiesmann F, Jahns R, Hadamek K, Arnolt C, Neubauer S, et al. Feedback inhibition of catecholamine release by two different alpha2-adrenoceptor subtypes prevents progression of heart failure. Circulation. 2002;106:2491–2496. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

